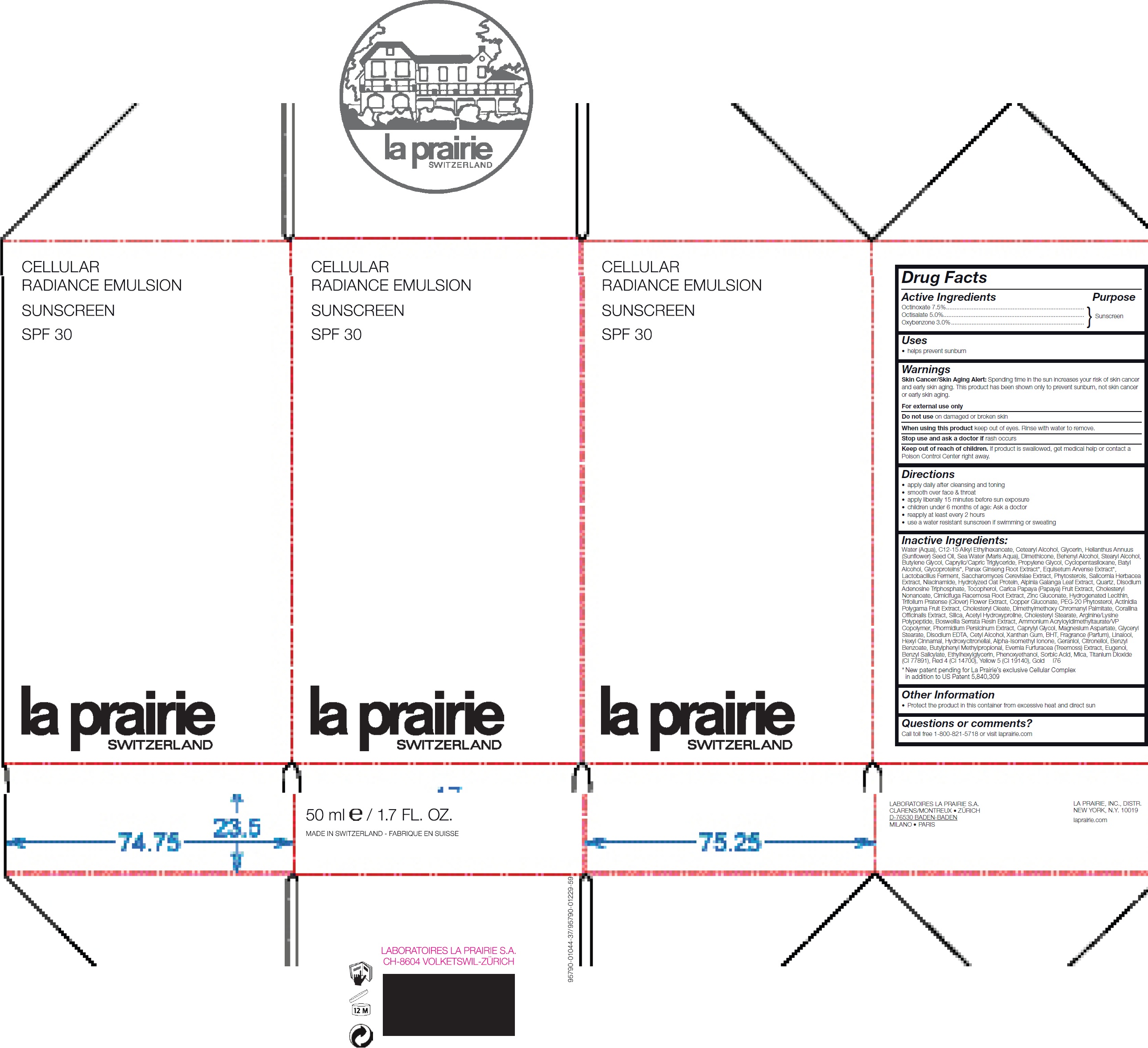
CELLULAR RADIANCE SUNSCREEN SPF 30- octinoxate, octisalate, oxybenzone emulsion
La Prairie, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CELLULAR RADIANCE SUNSCREEN SPF 30
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to preven sunburn, not skin cancer or early skin aging.
For external use only
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply daily after cleansing and toning
- smooth over face and throat
- apply liberally 15 minutes before sun exposure
- children under 6 months of age: Ask a doctor
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Inactive Ingredients
Water (Aqua), C12-15 Alkyl Ethylhexanoate, Cetearyl Alcohol, Glycerin, Helianthus Annuus (Sunflower) Seed Oil, Sea Water (Maris Aqua), Dimethicone, Behenyl Alcohol, Stearyl Alcohol, Butylene Glycol, Caprylic/Capric Triglyceride, Propylene Glycol, Cyclopentasiloxane, BatylAlcohol, Glycoproteins, Panax Ginseng Root Extract, Equisetum Arvense Extract, Lactobacillus Ferment, Saccharomyces Cerevisiae Extract, Phytosterols, Salicornia Herbacea Extract, Niacinamide, Hydrolyzed Oat Protein, Alpinia Galanga Leaf Extract, Quartz, Disodium Adenosine Triphosphate, Tocopherol, Carica Papaya (Papaya) Fruit Extract, Cholesteryl Nonanoate, Cimicifuga Racemosa Root Extract, Zinc Gluconate, Hydrogenated Lecithin, Trifolium Pratense (Clover) Flower Extract, Copper Gluconate, PEG-20 Phytosterol, Actinidia Polygama Fruit Extract, Cholesteryl Oleate, Dimethylmethoxy Chromanyl Palmitate, Corallina Ocinalis Extract, Silica, Acetyl Hydroxyproline, Cholesteryl Stearate, Arginine/Lysine Polypeptide, Boswellia Serrata Resin Extract, Ammonium Acryloyldimethyltaurate/VP Copolymer, Phormidium Persicinum Extract, Caprylyl Glycol, Magnesium Aspartate, Glyceryl Stearate, Disodium EDTA, Cetyl Alcohol, Xanthan Gum, BHT, Fragrance (Parfum), Linalool, Hexyl Cinnamal, Hydroxycitronellal, Alpha-Isomethyl Ionone, Geraniol, Citronellol, Benzyl Benzoate, Butylphenyl Methylpropional, Evernia Furfuracea (Treemoss) Extract, Eugenol, Benzyl Salicylate, Ethylhexylglycerin, Phenoxyethanol, Sorbic Acid, Mica, Titanium Dioxide ( CI 77891), Red 4 (CI 14700), Yellow 5 (CI 19140), Gold i76 New patent pending for La Prairie’s exclusive Cellular Complex in addition to US Patent 5,840,309
Questions or comments?
Call toll free 1-800-821-5718 or visit www.laprairie.com
CELLULAR RADIANCE EMULSION EMULSION CELLULAIRE RADIANCE SPF 30 la prairie SWITZERLAND CELLULAR RADIANCE EMULSION EMULSION CELLULAIRE RADIANCE SPF 30 la prairie SWITZERLAND
| CELLULAR RADIANCE SUNSCREEN SPF 30
octinoxate, octisalate, oxybenzone emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - La Prairie, Inc. (606554996) |