SKINTX UV PRO BROAD SPECTRUM SPF45- zinc oxide, octocrylene, octinoxate, octisalate lotion
Vivier Pharma Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
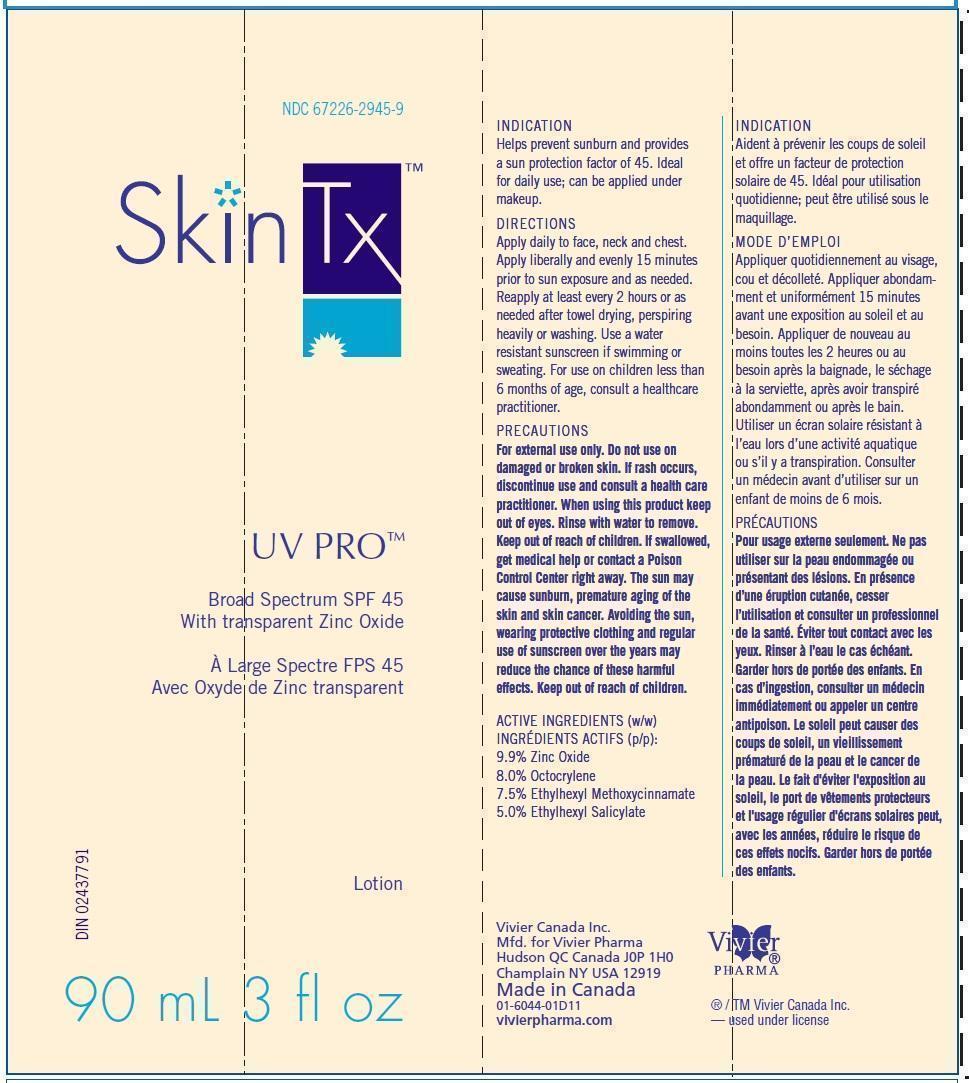
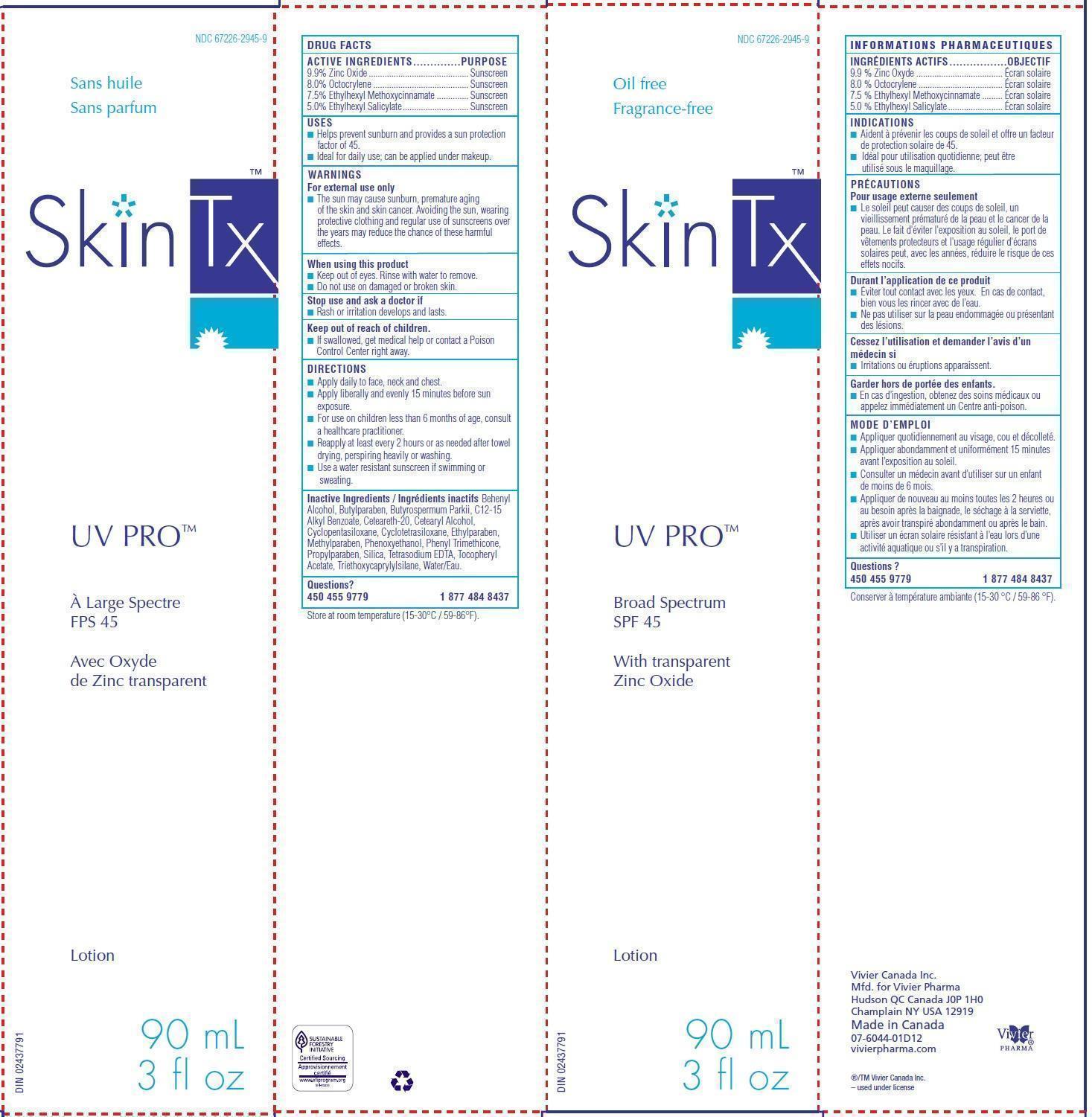
SkinTx UV Pro Broad Spectrum SPF45
ACTIVE INGREDIENTS
9.9% Zinc Oxide
8.0% Octocrylene
7.5% Ethylhexyl Methoxycinnamate
5.0% Ethylhexyl Salicylate
USES
■ Helps prevent sunburn and provides a sun protection factor of 45.
■ Ideal for daily use; can be applied under makeup.
For external use only
■ The sun may cause sunburn, premature aging of the skin and skin cancer. Avoiding the sun, wearing protective clothing and regular use of sunscreens over the years may reduce the chance of these harmful effects.
DIRECTIONS
■ Apply daily to face, neck and chest.
■ Apply liberally and evenly 15 minutes before sun exposure.
■ For use on children less than 6 months of age, consult a healthcare practitioner.
■ Reapply at least every 2 hours or as needed after towel drying, perspiring heavily or washing.
■ Use a water resistant sunscreen if swimming or sweating.
Inactive Ingredients
Behenyl Alcohol, Butylparaben, Butyrospermum Parkii, C12-15 Alkyl Benzoate, Ceteareth-20, Cetearyl Alcohol, Cyclopentasiloxane, Cyclotetrasiloxane, Ethylparaben, Methylparaben, Phenoxyethanol, Phenyl Trimethicone, Propylparaben, Silica, Tetrasodium EDTA, Tocopheryl Acetate, Triethoxycaprylylsilane, Water/Eau.
| SKINTX UV PRO BROAD SPECTRUM SPF45
zinc oxide, octocrylene, octinoxate, octisalate lotion |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Vivier Pharma Inc. (250996550) |