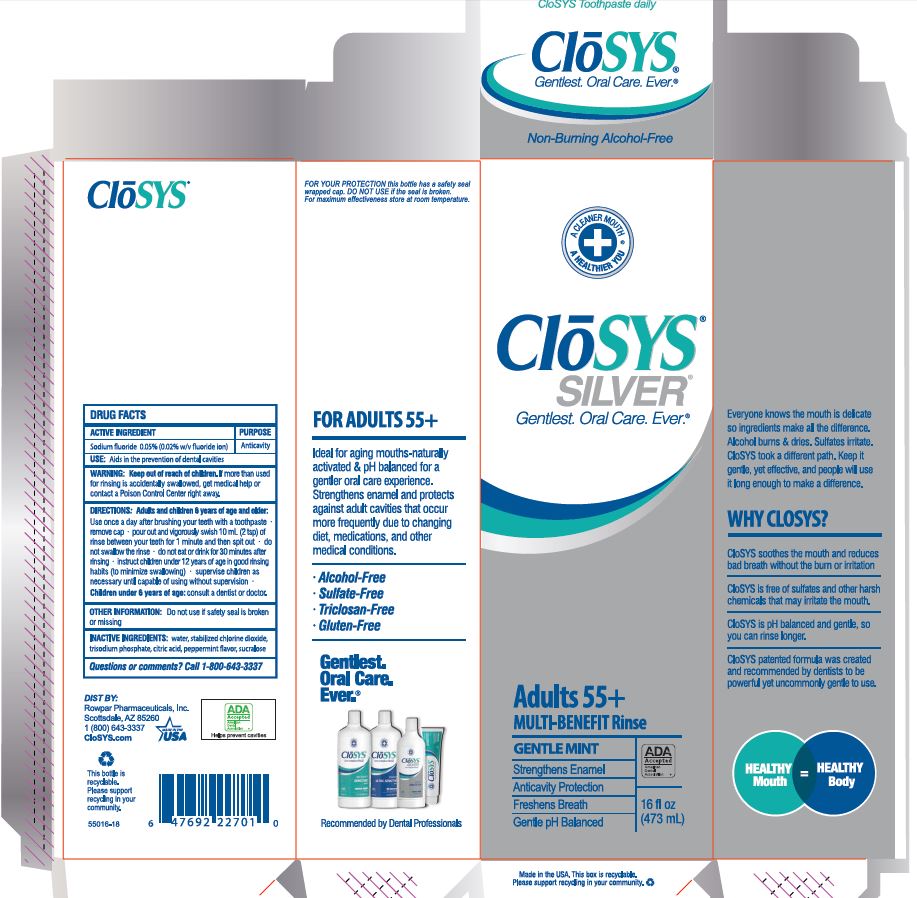
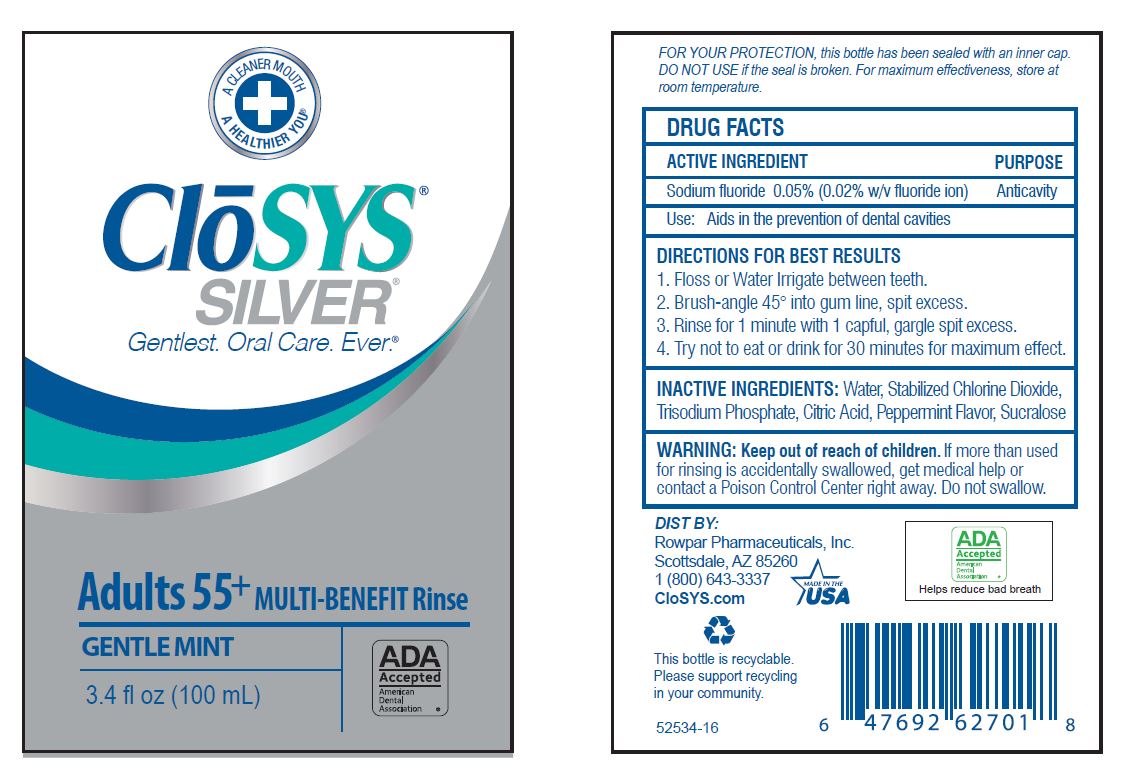
Label: CLOSYS FLUORIDE RINSE- sodium fluoride rinse
- NDC Code(s): 58578-1234-1, 58578-1234-2
- Packager: Rowpar Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
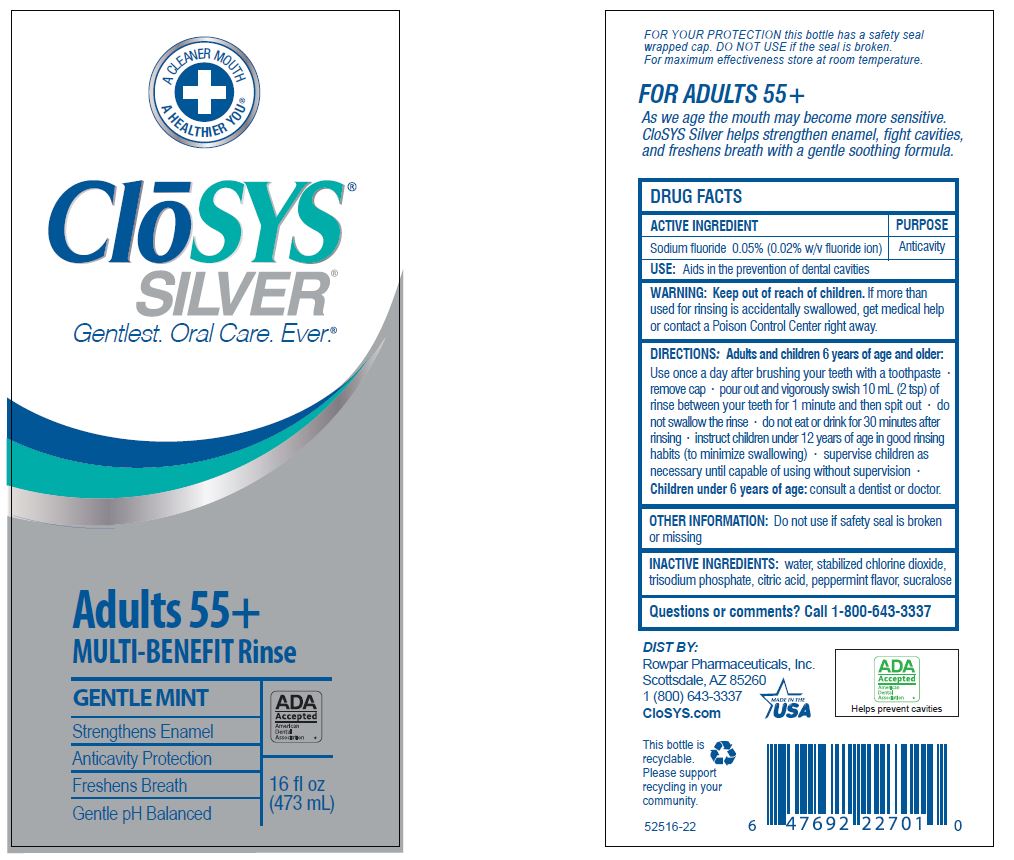
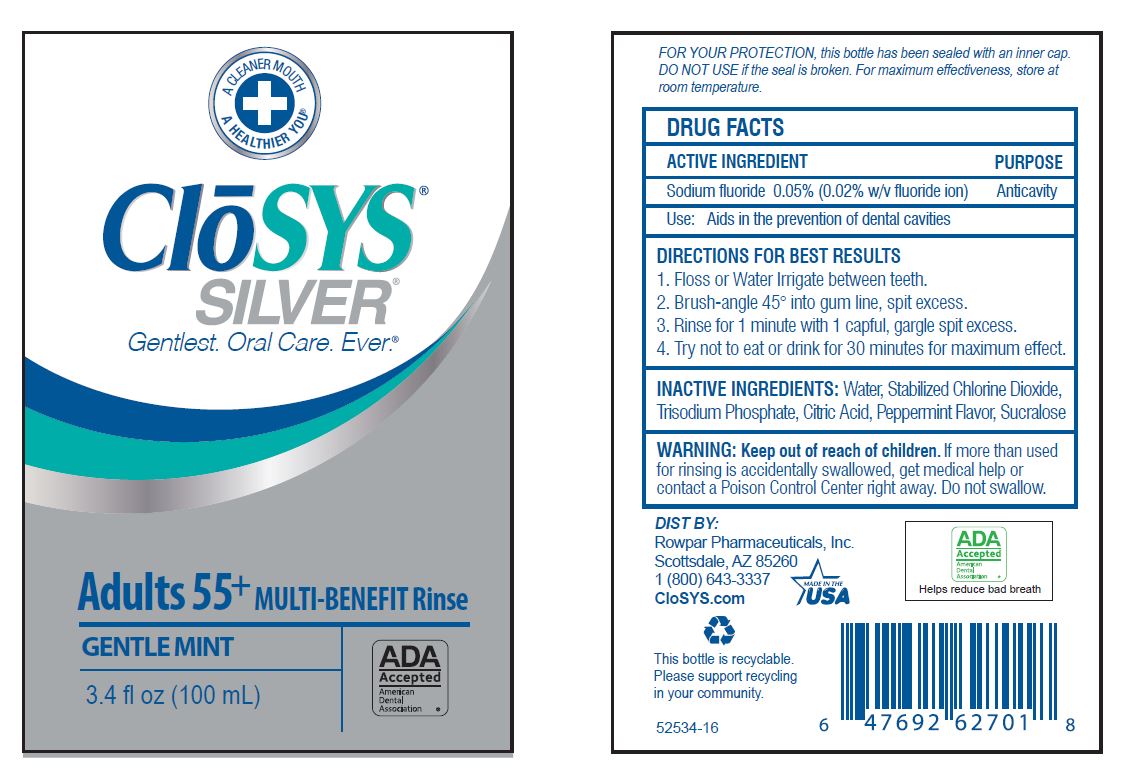
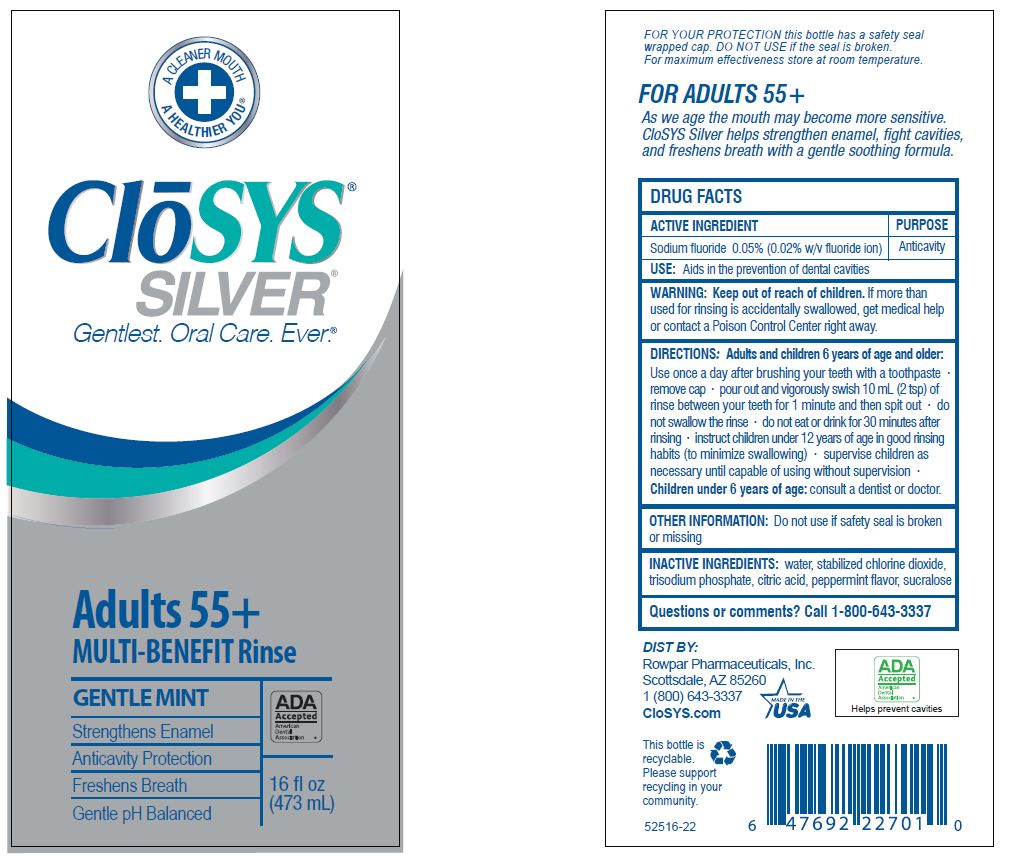
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- USE:
- WARNING:
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS: Adults and children 6 years of age and older:
Use once a day after brushing your teeth with a toothpaste · remove cap · pour out and vigorously swish 10 ml (2 tsp) of rinse between your teeth for 1 minute and then spit out · do not swallow the rinse · do not eat or drink for 30 minutes after rinsing • instruct children under 12 years of age in good rinsing habits (to minimize swallowing) · supervise children as necessary until capable of using without supervision ·
Children under 6 years of age: consult a dentist or doctor. - OTHER INFORMATION:
- INACTIVE INGREDIENTS:
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
CLOSYS FLUORIDE RINSE
sodium fluoride rinseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58578-1234 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CHLORINE DIOXIDE (UNII: 8061YMS4RM) SODIUM PHOSPHATE, TRIBASIC, DODECAHYDRATE (UNII: B70850QPHR) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PEPPERMINT (UNII: V95R5KMY2B) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58578-1234-1 1 in 1 BOX 02/18/2016 1 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:58578-1234-2 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/18/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 02/18/2016 Labeler - Rowpar Pharmaceuticals, Inc. (783704661)