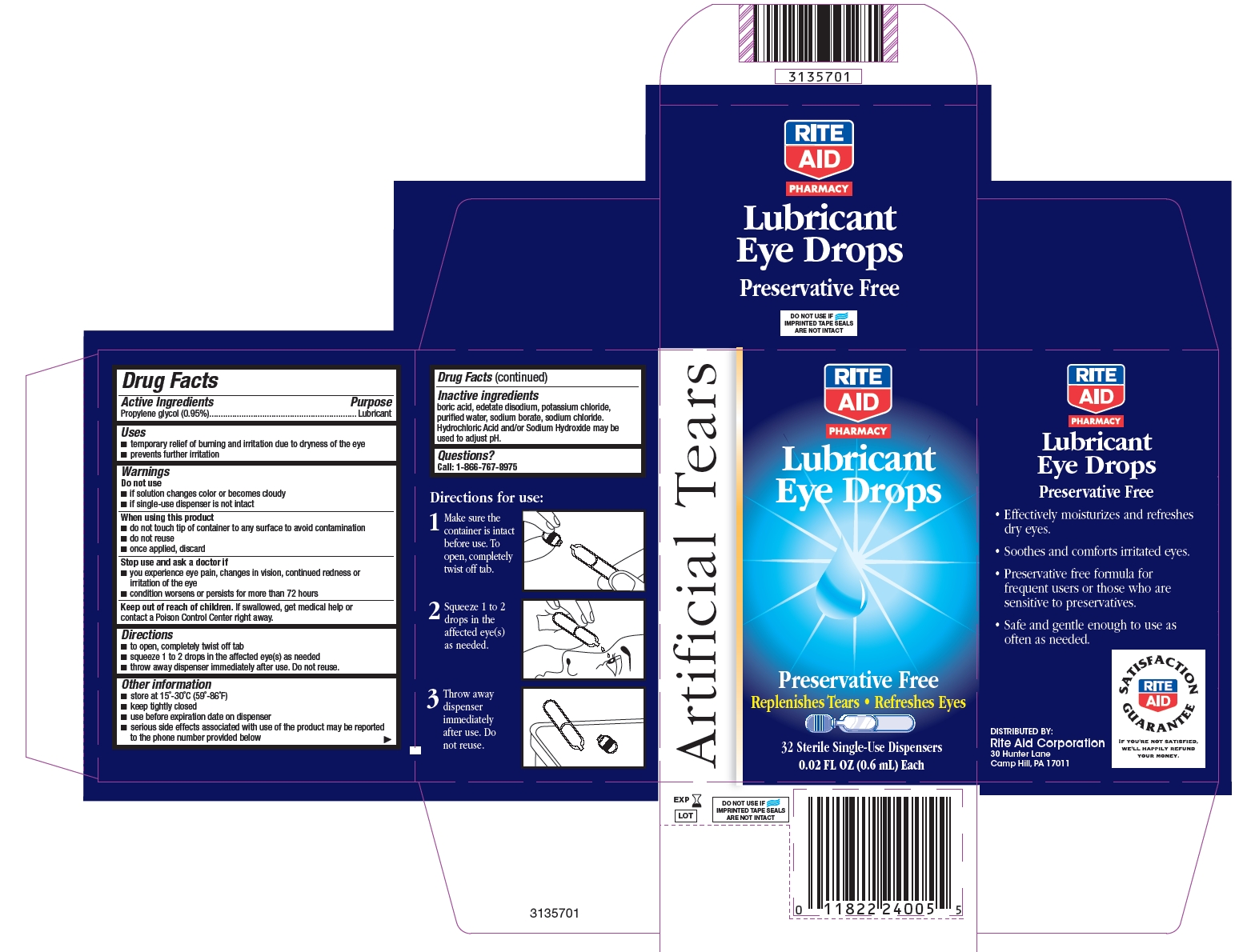
ARTIFICIAL TEARS- propylene glycol solution/ drops
Rite Aid Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- temporary relief of burning and irritation due to dryness of the eye
- •
- prevents further irritation
Warnings
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- do not reuse
- •
- once applied, discard
Directions
- •
- to open, completely twist off tab
- •
- squeeze 1 to 2 drops in the affected eye(s) as needed
- •
- throw away dispenser immediately after use. Do not reuse.
Other information
- •
- store at 15°-30°C (59°-86°F)
- •
- keep tightly closed
- •
- use before expiration date on dispenser
- •
- serious side effects associated with use of the product may be reported to the phone number provided below
Inactive ingredients
boric acid, edetate disodium, potassium chloride, purified water, sodium borate, sodium chloride. Hydrochloric Acid and/or Sodium Hydroxide may be used to adjust pH.
Package/Label Principal Display Panel
ARTIFICIAL TEARS
LUBRICANT EYE DROPS
Preservative Free
Replenishes Tears • Refreshes Eyes
- •
- Effectively moisturizes and refreshes dry eyes.
- •
- Soothes and comforts irritated eyes.
- •
- Preservative free formula for frequent users or those who are sensitive to preservatives.
- •
- Safe and gentle enough to use as often as needed.
DISTRIBUTED BY:
Rite Aid Corporation
30 Hunter Lane
Camp Hill, PA 17011
DO NOT USE IF IMPRINTED TAPE SEALS ARE NOT INTACT
| ARTIFICIAL TEARS
propylene glycol solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Rite Aid Corporation (014578892) |
| Registrant - Baush & Lomb Incorporated (196603781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 114406598 | MANUFACTURE(11822-2400) | |
Revised: 9/2010
Document Id: 197d15d8-e7b7-4013-a806-c1608c421949
Set id: 1a8640de-e2bb-48e5-b664-e0c157a2337d
Version: 2
Effective Time: 20100901
Rite Aid Corporation