PHOS-AID- phosphorus injection
Butler Animal Health Supply LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Henry Schein Animal Health
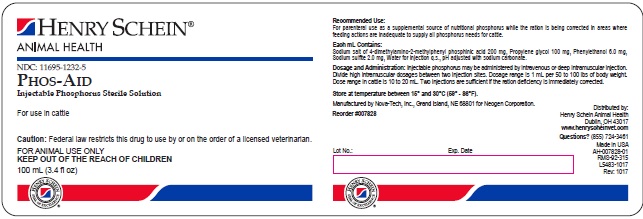
Phos-Aid
Injectable Phosphorous Sterile Solution
Recommended Use:
For parenteral use as a supplemental source of nutritional phosphorus while the ration is being corrected in areas where feeding actions are inadequate to supply all phosporus needs for cattle.
Each mL Contains:
Sodium salt of 4-dimethylamino-2-methylphenyl phosphinic acid 200 mg. Propylene glycol 100 mg. Phenylethanol 6.0 mg. Sodium sulfite 2.0 mg. Water for injection q.s., pH adjusted with sodium carbonate.
Dosage and Administration:
Injectable phosphorous may be administered by intravenous or deep intramuscular injection. Divide high intramuscular dosages between two injection sites. Dosage range is 1 mL per 50 to 100 lbs of body weight. Dose range in cattle is 10 to 20 mL. Two injections are sufficient if the ration deficiency is immediately corrected.
Manufactured by Nova-Tech, Inc., Grand Island, NE 68801 for Neogen Corporation
Reorder #007828
Distributed by:
Henry Schein Animal Health
Dublin, OH 43017
www.henryscheinvet.com
Questions? (855) 724-3461
Made in USA
AH-007828-01
RMS-92-315
L5483-1017
Rev: 1017
Lot No.
Exp. Date
| PHOS-AID
phosphorus injection |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Butler Animal Health Supply LLC (603750329) |
| Registrant - Neogen Corporation (042125879) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nova-Tech, Inc | 196078976 | api manufacture, manufacture | |