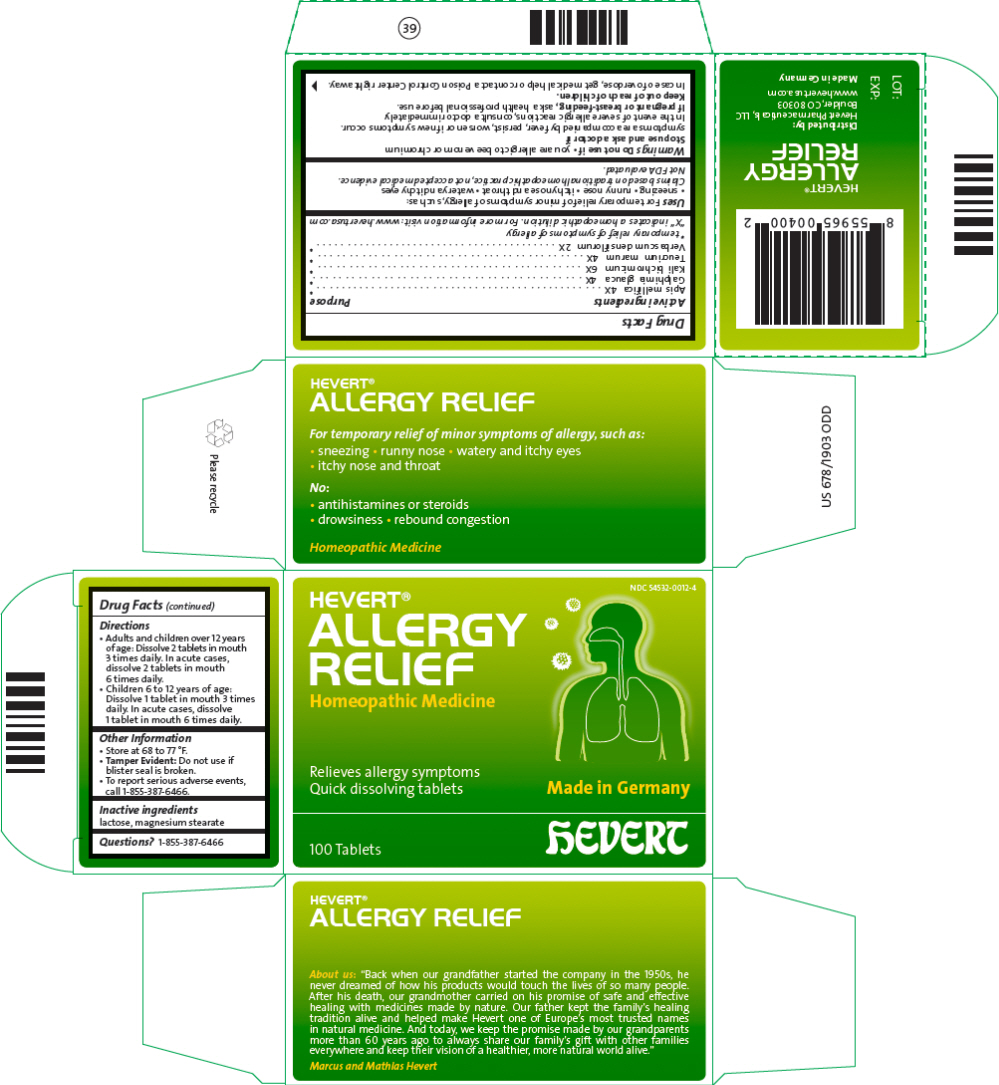
HEVERT ALLERGY RELIEF- apis mellifera, galphimia glauca whole, potassium dichromate, teucrium marum top, and verbascum densiflorum flowering top tablet
Hevert Pharmaceuticals LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
HEVERT® ALLERGY RELIEF
Uses
For temporary relief of minor symptoms of allergy, such as:
- sneezing
- runny nose
- itchy nose and throat
- watery and itchy eyes
Claims based on traditional homeopathic practice, not accepted medical evidence.
Not FDA evaluated.
Warnings
Directions
- Adults and children over 12 years of age: Dissolve 2 tablets in mouth 3 times daily. In acute cases, dissolve 2 tablets in mouth 6 times daily.
- Children 6 to 12 years of age: Dissolve 1 tablet in mouth 3 times daily. In acute cases, dissolve 1 tablet in mouth 6 times daily.
| HEVERT
ALLERGY RELIEF
apis mellifera, galphimia glauca whole, potassium dichromate, teucrium marum top, and verbascum densiflorum flowering top tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Hevert Pharmaceuticals LLC (078647622) |
Revised: 2/2020
Document Id: 3fef1842-4d6f-446c-a037-5fcdd9a1e65d
Set id: 17b34bf1-7c6b-47d3-8e6a-31e911b39d43
Version: 7
Effective Time: 20200214
Hevert Pharmaceuticals LLC