Label: MEDACTIVE PATIENT FRIENDLY STANNOUS FLUORIDE RINSE- stannous fluoride kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 49741-1006-2, 49741-1006-3 - Packager: Integrate Oral Care, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
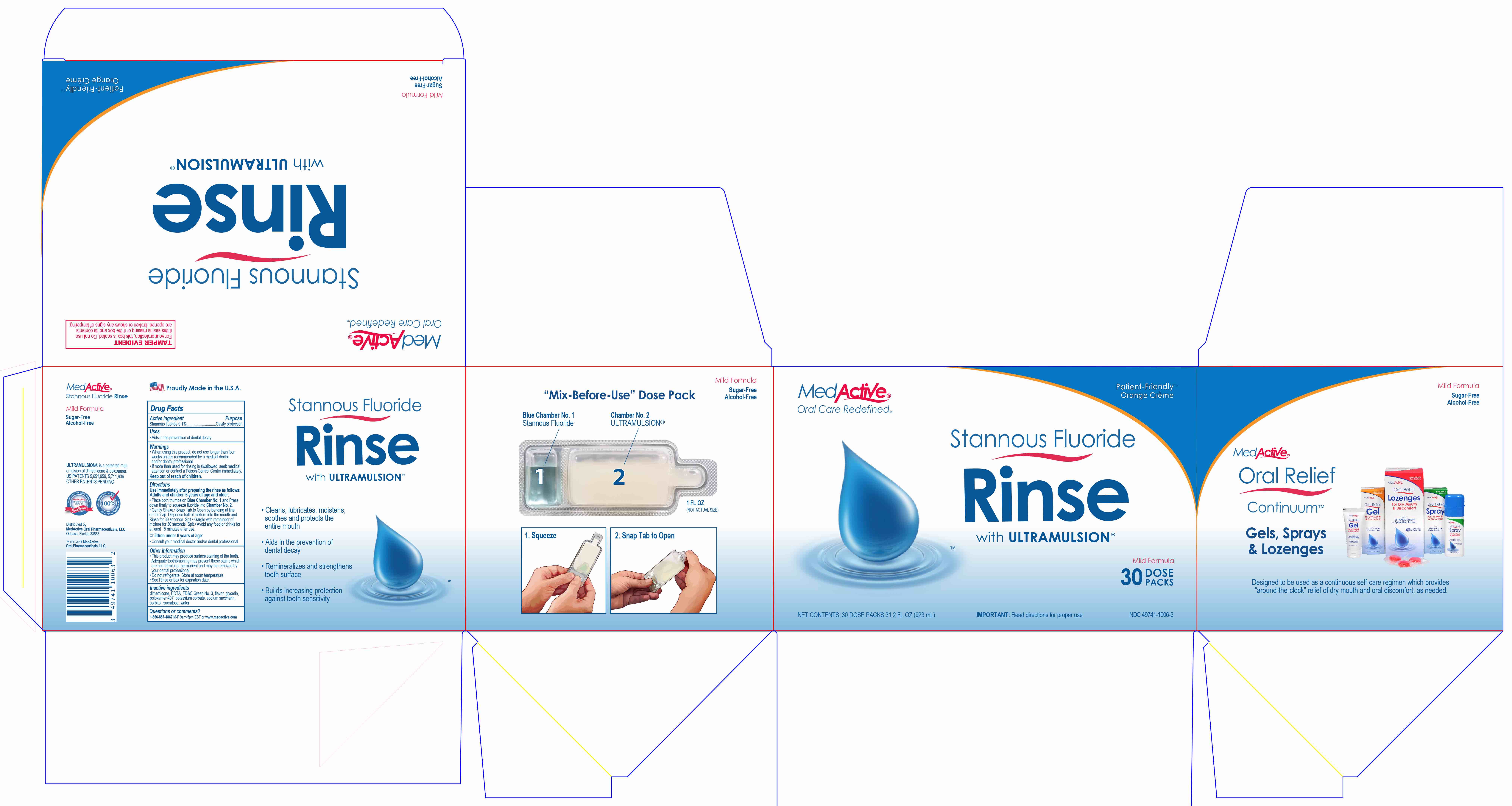
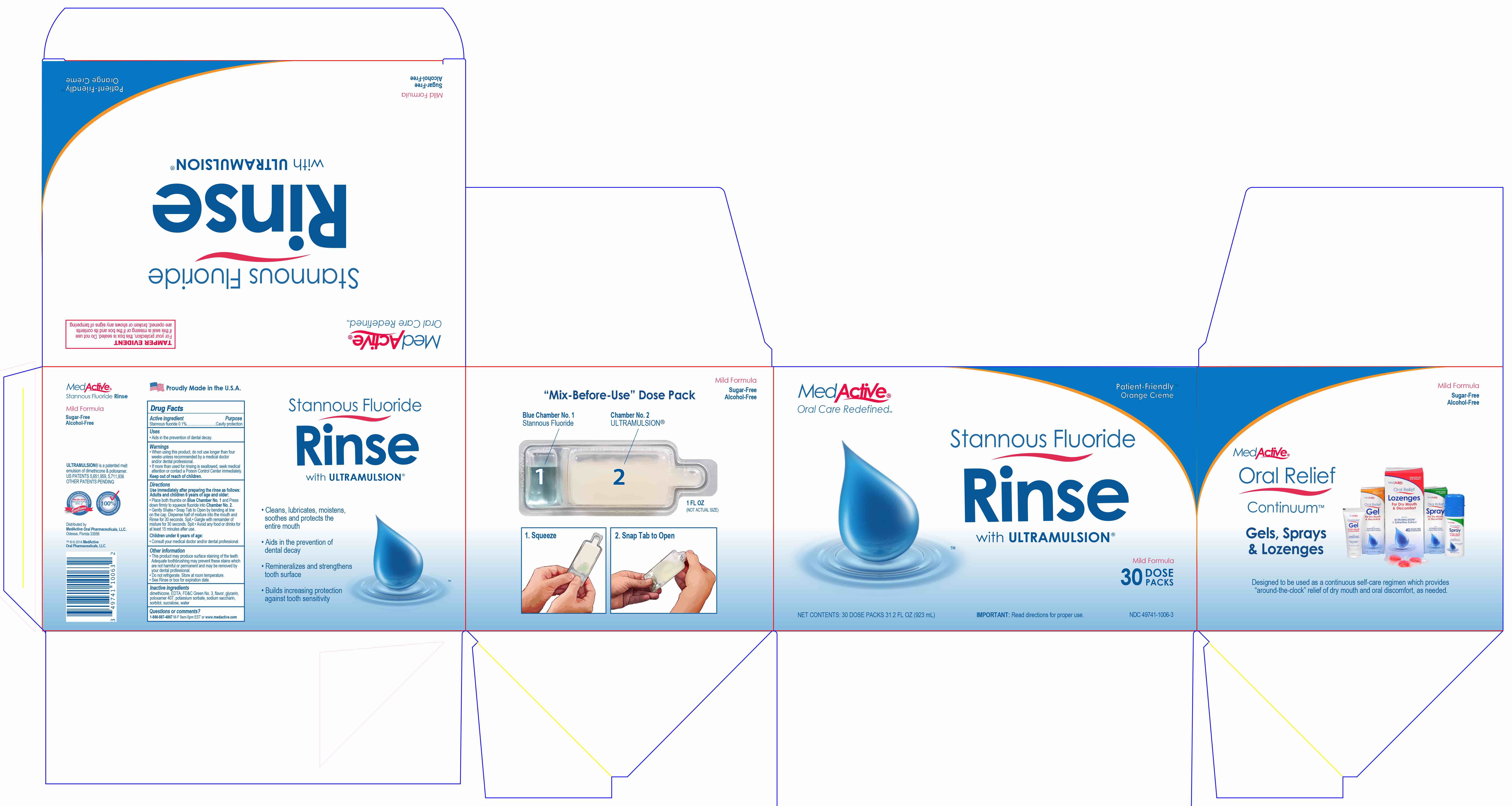
Directions
Use immediately after preparing the rinse as follows:
Adults and children 6 years or age and older:
- Place both thumbs on Blue Chamber No. 1 and Press down firmly to squeeze fluoride into Chamber No. 2.
- Gently Shake.
- Snap Tab to Open by bending at line on the cap. Dispense half of mixture into the mouth and Rinse for 30 seconds. Spit.
- Gargle with remainder of mixture for 30 seconds. Spit.
- Avoid any food or drinks for at least 15 minutes after use.
Children under 6 years of age:
- Consult your medical doctor and/or dental professional.
- WARNINGS
- INACTIVE INGREDIENT
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- Prinicpal Display Panel - 30 Dose Packs
- Principal Display Panel - 10 Dose Packs
-
INGREDIENTS AND APPEARANCE
MEDACTIVE PATIENT FRIENDLY STANNOUS FLUORIDE RINSE
stannous fluoride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49741-1006 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49741-1006-2 10 in 1 BOX 07/31/2014 1 1 in 1 CARTON; Type 0: Not a Combination Product 2 NDC:49741-1006-3 30 in 1 BOX 07/31/2014 2 1 in 1 CARTON; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 KIT 3.7 mL Part 2 1 KIT 27 mL Part 1 of 2 CHAMBER NO. 1
stannous fluoride concentrateProduct Information Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 6.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 1 mL in 1 mL FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 3.7 mL in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 07/31/2014 Part 2 of 2 CHAMBER NO. 2
stannous fluoride diluent emulsionProduct Information Route of Administration ORAL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) EDETIC ACID (UNII: 9G34HU7RV0) POLOXAMER 407 (UNII: TUF2IVW3M2) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) Product Characteristics Color Score Shape Size Flavor ORANGE (Orange Crème with ULTRAMULSION®) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 27 mL in 1 KIT; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 07/31/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 07/31/2014 Labeler - Integrate Oral Care, LLC. (063540457)


 MedActive
MedActive
 MedActive
MedActive