GELX- dressing, wound and burn, hydrogel w/drug and/or biologic
Key Therapeutics, LLC
----------
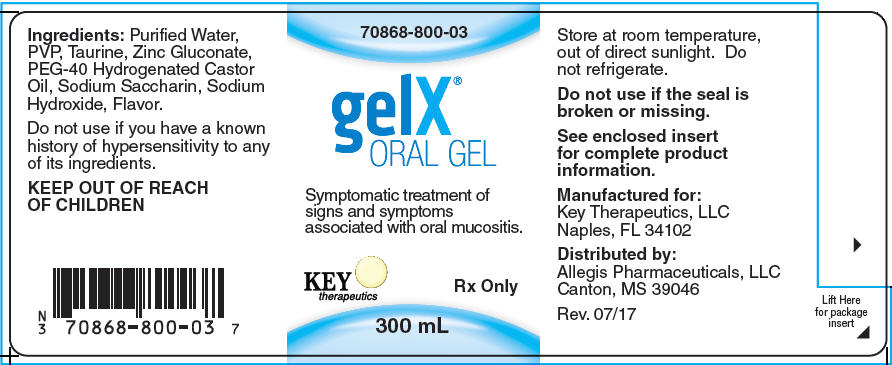
GelX® Oral Gel
Ingredients
Purified Water, PVP, Taurine, Zinc Gluconate, PEG-40 Hydrogenated Castor Oil, Sodium Saccharin, Sodium Hydroxide, Flavor.
Indications
GelX® ORAL GEL has a mechanical action indicated for the management of pain and relief of pain by adhering to the mucosal surface of the mouth, soothing the lesions caused by various etiologies, including: Oral mucositis/stomatitis (may be caused by chemotherapy and/or radiation therapy), irritation due to oral surgery, and traumatic ulcers caused by braces or ill-fitting dentures, or disease. Also indicated for the treatment of diffuse aphthous ulcers.
Contraindications
GelX® is contraindicated in any patient with a known history of hypersensitivity to any of its ingredients.
Special Precautions for use
Avoid eating or drinking for at least one hour after use. Do not use if the product seal is damaged or missing. If no improvement is noticed after 7 days, consult a physician.
Warnings
Federal law restricts GelX® to sale by or on the order of a physician or properly licensed practitioner.
Interactions with other medicinal products and other forms of interaction
There are no known interactions with medicinal or other products.
DIRECTIONS FOR USE
Use 15mL of product three (3) times a day, or as needed. Rinse around the mouth for at least one minute, or as long as possible to coat all the oral mucosa thoroughly. SWISH and SPIT OUT completely. DO NOT SWALLOW. Do not eat or drink for at least one hour following treatment. If GelX® is swallowed accidently, no adverse effects are anticipated.
Side effects
At the time of producing this leaflet there have been no reported side effects with GelX®, however the product is not recommended for use in patients with a known or suspected allergy to any of the product's ingredients.
Call your doctor about side effects. To report Suspected Adverse Reactions, contact Key Therapeutics, LLC at 1-888-981-8337, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdose
At the time of producing this leaflet no cases of overdose have been reported. However, no serious adverse effects should be expected from ingestion of up to one bottle of GelX®.
Please note: The gel may become a little darker and thicker over time, but this does not affect its efficacy or safety. Do not use after the 'Best Before' date shown on the bottle.
KEEP OUT OF THE REACH OF CHILDREN
Rx Only
Manufactured for: Key Therapeutics, LLC, Naples, FL 34102
Distributed by: Allegis Pharmaceuticals, LLC
Canton, MS 39046
Last Revision: July 2017
GelX® is produced under license from BMG Pharma Srl, Milan, Italy.
| GELX
dressing, wound and burn, hydrogel w/drug and/or biologic |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Key Therapeutics, LLC (080318791) |