CONQUEST- chloroxylenol solution
KAY CHEMICAL CO.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
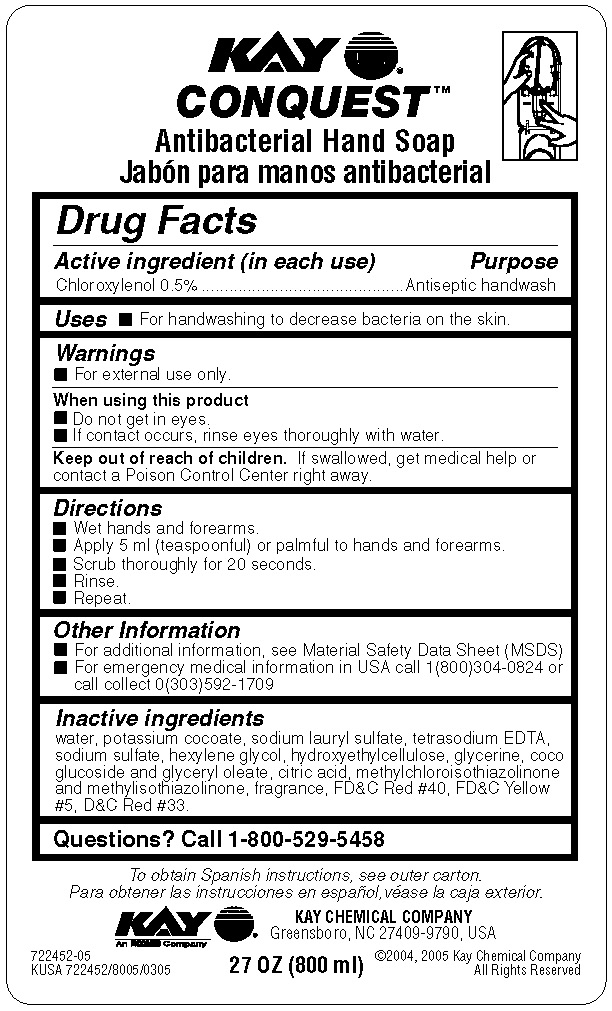
Drug Facts
Directions
- Wet hands and forearms.
- Apply 5 ml (teaspoonful) or palmful to hands and forearms.
- Scrub thoroughly for 20 seconds.
- Rinse.
- Repeat.
Other Information
- For additional information, see Material Safety Data Sheet (MSDS)
- For emergency medical information in USA call 1(800)304-0824 or call collect 0(303)592-1709
Inactive ingredients water, potassium cocoate, sodium lauryl sulfate, tetrasodium EDTA, sodium sulfate, hexylene glycol, hydroxyethylcellulose, glycerine, coco glucoside and glyceryl oleate, citric acid, methylchloroisothiazolinone and methylisothiazolinone, fragrance, FDC Red #40, FDC Yellow #5, DC Red #33
| CONQUEST
chloroxylenol solution |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - KAY CHEMICAL CO. (003237021) |
Revised: 3/2018
Document Id: bf50f61c-0c8a-4b7d-8af8-c008a57a7f02
Set id: 17244a4a-6383-4df0-af89-9993e7f0e8ed
Version: 2
Effective Time: 20180312
KAY CHEMICAL CO.