Label: GREENGLO- lissamine green strip
- NDC Code(s): 17238-920-11, 17238-920-99
- Packager: HUB Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Product Facts
- INDICATIONS:
-
DIRECTIONS FOR USE:
For best viewing and patient comfort, the Lissamine Green impregnated tip should be moistened with one or two drops of sterile irrigating or saline solution before application. Apply moistened tip to conjunctiva or fornix as required. It is recommended that the patient blink several times after application.
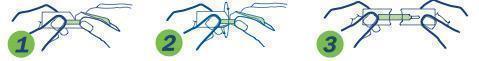
1. Hold package in middle and tear down toward sterile strip
2. Turn over and tear down from other side, taking care not to tear the sterile strip.
3. Grasp each end of package firmly as shown, without touching the sterile strip, and pull apart.
- NOTE:
- HOW SUPPLIED:
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Contacare Ophthalmics & Diagnositics (EOU)
Block No. 310, Village Sim of Dabhasa, Taluka: Padra,
Dist.: Vadodara - 391 440, Gujarat, India.
Mfg. Lic. No. G/1197
Manufactured for, and distributed by:
HUB Pharmaceuticals, LLC
Rancho Cucamonga, CA 91730
www.hubrx.com
European Representative:
MDT J.Zych, A. Budyn Sp j.
ul. Skosna 12A, 30-383 Krakow, Poland
- Representative Packaging:
-
INGREDIENTS AND APPEARANCE
GREENGLO
lissamine green stripProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17238-920 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIGHT GREEN SF YELLOWISH (UNII: 3F7BHA64Z0) (LIGHT GREEN SF YELLOWISH - UNII:3F7BHA64Z0) LIGHT GREEN SF YELLOWISH 1.5 mg in 1.5 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17238-920-11 100 in 1 BOX 04/01/2012 1 NDC:17238-920-99 1.5 mg in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/01/2012 Labeler - HUB Pharmaceuticals, Inc. (611747945) Establishment Name Address ID/FEI Business Operations OMNI LENS PRIVATE LIMITED 862170057 manufacture(17238-920) Establishment Name Address ID/FEI Business Operations Madhu Instruments Pvt.Ltd. 915827852 manufacture(17238-920)




