Label: LAXATIVE PILLS MAXIMUM STRENGTH- sennosides tablet
- NDC Code(s): 37808-492-24
- Packager: H E B
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
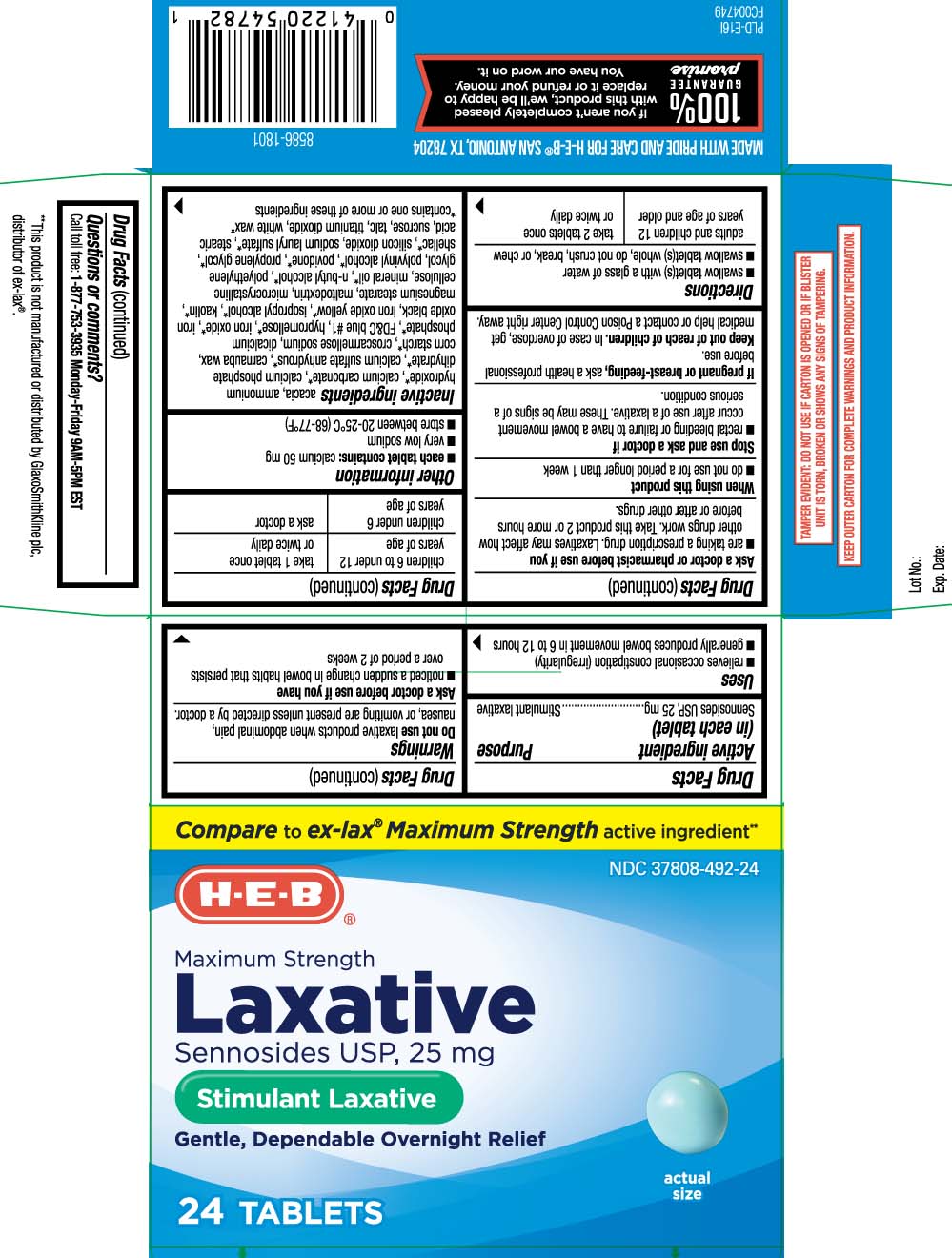
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist before use if you
- are taking a prescription drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
- Directions
- Other information
-
Inactive ingredients
acacia, ammonium hydroxide*, calcium carbonate*, calcium phosphate dehydrate*, calcium sulfate anhydrous*, carnauba wax, corn starch*, croscarmellose sodium, dicalcium phosphate*, FD&C blue #1, hypromellose*, iron oxide*, iron oxide black, iron oxide yellow*, isopropyl alcohol*, kaolin*, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil*, n-butyl alcohol*, polyethylene glycol, polyvinyl alcohol*, povidone*, propylene glycol*, shellac*, silicon dioxide*, sodium lauryl sulfate*, stearic acid, sucrose, talc, titanium dioxide, white wax*
*contains one or more of these ingredients
- Questions or comments?
-
Principal Display Panel
Compare to ex-lax® Maximum Strength active ingredient**
Maximum Strength
Laxative Pills
Sennosides USP, 25 mg
Stimulant Laxative
Gentle, Dependable, Overnight Relief
TABLETS
**This product is not manufactured or distributed by GlaxoSmithKline plc, distributor of Ex-Lax®.
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
MADE WITH PRIDE AND CAEW FOR H-E-B® SAN ANTONIO, TX 78204
- Product Label
-
INGREDIENTS AND APPEARANCE
LAXATIVE PILLS MAXIMUM STRENGTH
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-492 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 25 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) AMMONIA (UNII: 5138Q19F1X) CALCIUM CARBONATE (UNII: H0G9379FGK) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ISOPROPYL ALCOHOL (UNII: ND2M416302) KAOLIN (UNII: 24H4NWX5CO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WHITE WAX (UNII: 7G1J5DA97F) Product Characteristics Color blue Score no score Shape ROUND Size 10mm Flavor Imprint Code TCL083;S25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-492-24 24 in 1 CARTON 04/30/2016 04/30/2025 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 04/30/2016 04/30/2025 Labeler - H E B (007924756)