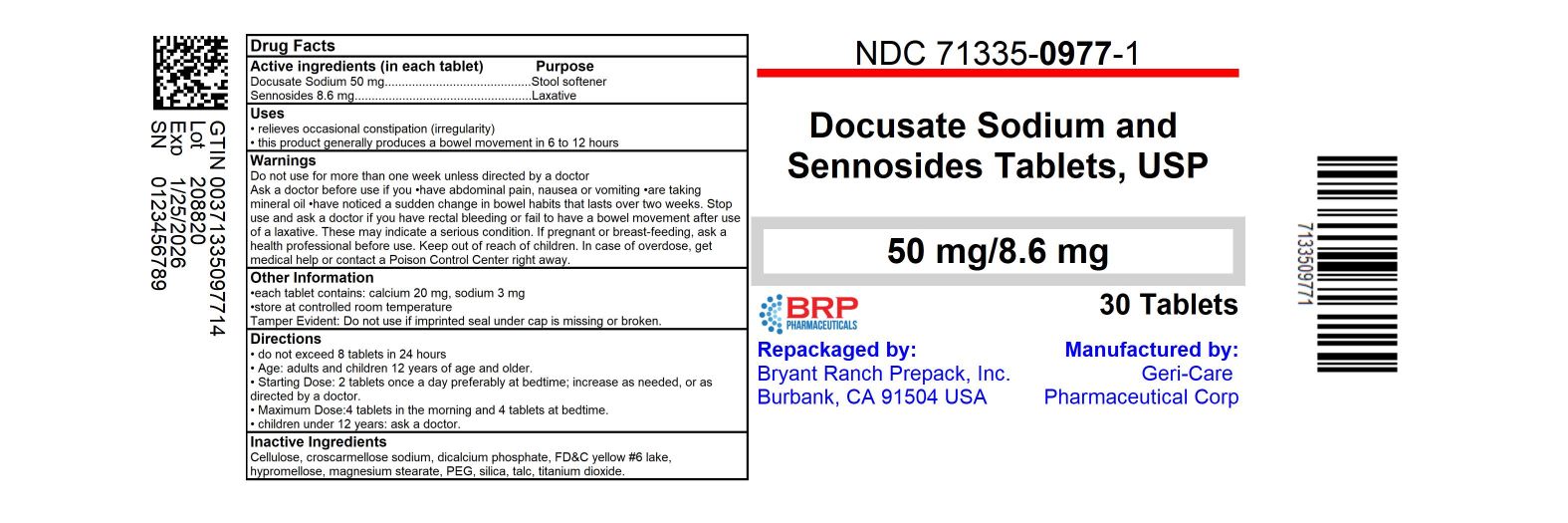
SENNA PLUS- sennosides and docusate sodium tablet, film coated
Bryant Ranch Prepack
----------
gc455
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use for more than 1 week unless directed by a doctor
Ask a doctor before use if you -have abdominal pain, nausea or vomiting -are taking mineral oil -have noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if -you have no bowel movement within 12 hours -you have rectal bleeding. these could signs of a serious condition.
if pregnant or breast-feeding, ask a health professional before use.
Directions
• do not exceed 8 tablets in 24 hours
| Age
| Starting Dose
| Maximum Dose
|
|---|---|---|
| adults and children 12 years of age and older
| 2 tablets once a day preferably at bedtime; increase as needed, or as directed by a doctor
| 4 tablets in the morning and 4 tablets at bedtime
|
| children under 12 years
| ask a doctor
|
Inactive ingredients
cellulose, croscarmellose sodium, dicalcium phosphate, FD and C yellow no. 5 (tartrazine), FD and C yellow no. 6, hypromellose, magnesium silicate, magnesium stearate, mineral oil, PEG, sodium benzoate, sodium lauryl sulfate, starch, stearic acid, titanium dioxide, triacetin
HOW SUPPLIED
NDC: 71335-0977-1: 30 Tablets in a BOTTLE
NDC: 71335-0977-2: 120 Tablets in a BOTTLE
NDC: 71335-0977-3: 60 Tablets in a BOTTLE
NDC: 71335-0977-4: 90 Tablets in a BOTTLE
NDC: 71335-0977-5: 100 Tablets in a BOTTLE
NDC: 71335-0977-6: 28 Tablets in a BOTTLE
NDC: 71335-0977-7: 56 Tablets in a BOTTLE
NDC: 71335-0977-8: 14 Tablets in a BOTTLE
NDC: 71335-0977-9: 20 Tablets in a BOTTLE
| SENNA PLUS
sennosides and docusate sodium tablet, film coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(71335-0977) , RELABEL(71335-0977) | |