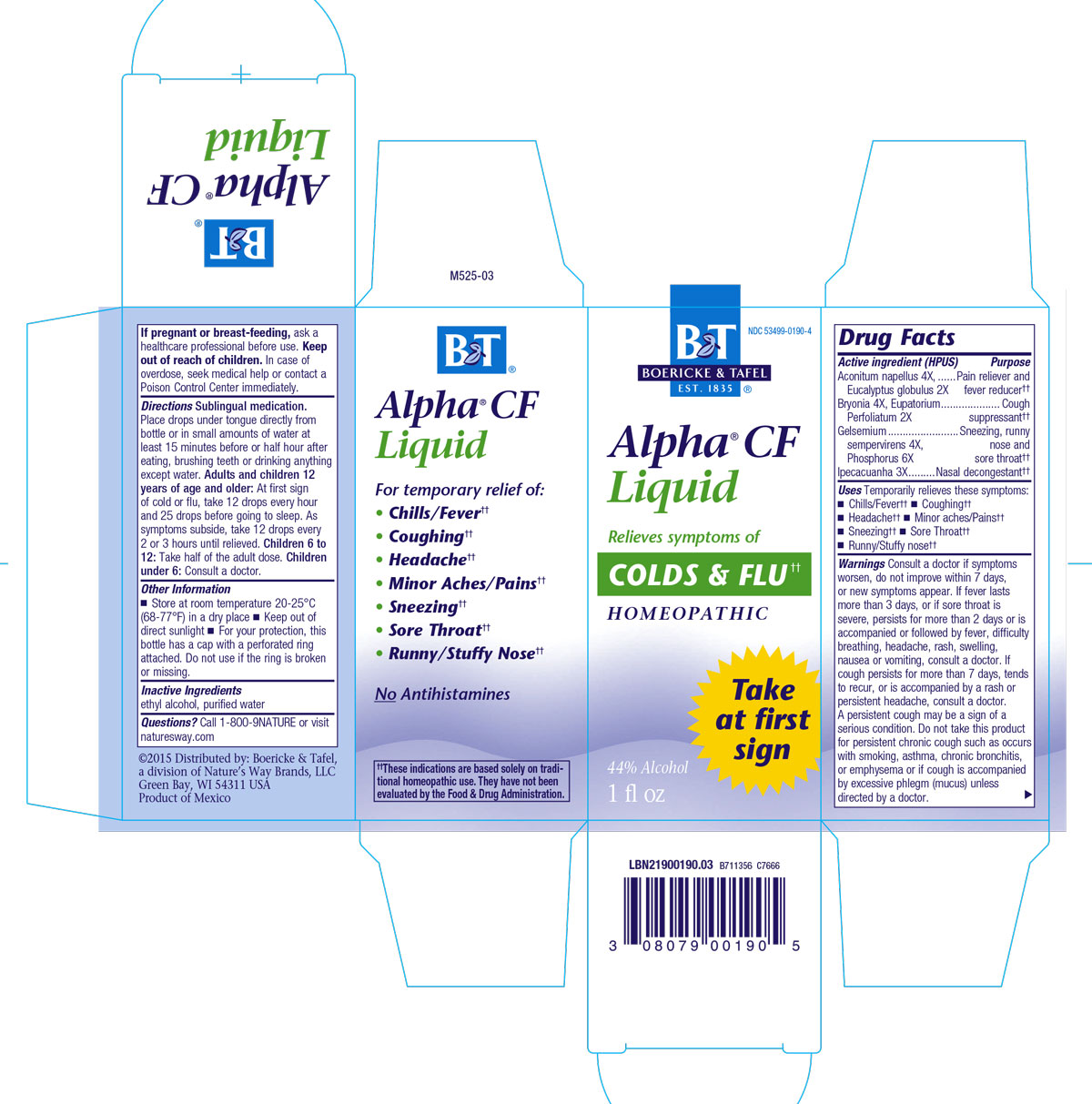
ALPHA CF- aconitum napellus, bryonia alba root, eupatorium perfoliatum flowering top, gelsemium sempervirens root, ipecac, phosphoruseucalyptus globulus leaf liquid
Schwabe North America, Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Alpha CF Liquid
Broecke and Tafel Brand
Active Ingredients:
Acontium Napellus 4X
Bryonia 4X
Eupatorium Perfoliatum 2X
Gelsemium Sempervirens 4X
Ipecacuanha 3X
Phosphorus 6X
Eucalyptus Globulus 2X
Dosage and Administration:
Recommendations:Sublingual medication.
Place drops under tongue directly from bottle or in small amount of water at least 15 minutes before or half hour after eating, brushing teeth, or drinking anything except water.
Adults and children 12 years of age and older:
At first sign of cold or flu, 12 drops every hour and 25 drops before going to sleep. As symptoms subside, 12 drops every 2 or 3 hours until relieved.
Children 6 to 12:
Half of the adult dose.
Children under 6:
Consult a doctor.
Warnings: Consult a doctor if symptoms worsen, do not improve within 7 days, or new symptoms appear.
If fever lasts more than 3 days, or if sore throat is severe, persists for more than 2 days, or is accompanied or followed by fever, difficulty breathing, headache, rash, swelling, nausea, or vomiting, consult a doctor.
If cough persists for more than 7 days, tends to recur, or is accompanied by a rash or persistent headache, consult a doctor.
A persistent cough may be a sign of a serious condition.
Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
Indications and Usage:
Uses: Temporarily relieves these symptions: fever/chills, sneezing, runny/stuffy nose, coughing, sore throat, headache, minor aches and pains that may accompany colds or flu.
Ask the Doctor:
Consult a doctor if symptoms worsen, do not improve within 7 days, or new symptoms appear.
If fever lasts more than 3 days, or if sore throat is severe, persists for more than 2 days, or is accompanied or followed by fever, difficulty breathing, headache, rash, swelling, nausea, or vomiting, consult a doctor.
If cough persists for more than 7 days, tends to recur, or is accompanied by a rash or persistent headache, consult a doctor.
A persistent cough may be a sign of a serious condition.
Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema or if cough is accompanied by excessive phlegm (mucus) unless directed by a doctor.
| ALPHA CF
aconitum napellus, bryonia alba root, eupatorium perfoliatum flowering top, gelsemium sempervirens root, ipecac, phosphoruseucalyptus globulus leaf liquid |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Schwabe North America, Inc (831153908) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Schwabe Mexico, S.A. de C.V. | 812805901 | manufacture(53499-0190) | |