AMPICILLIN- ampicillin capsule
AMPICILLIN- ampicillin suspension
Par Pharmaceutical
----------
AMPICILLIN CAPSULES, USP 250 mg and 500 mg
AMPICILLIN FOR ORAL SUSPENSION, USP 125 mg/5 mL and 250 mg/5 mL
Rx Only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin capsules, Ampicillin for Oral Suspension and other antibacterial drugs, Ampicillin Capsules and Ampicillin for Oral Suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Ampicillin trihydrate is a semisynthetic penicillin derived from the basic penicillin nucleus, 6-aminopenicillanic acid. Ampicillin is designated chemically as (2S, 5R, 6R)-6-[(R)-2-Amino-2-phenylacetamido]-3, 3-dimethyl-7-oxo-4-thia-l-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate.
It has the following chemical structure:
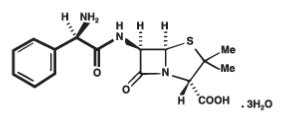
The molecular formula is C16H19N3O4S.3H2O, and the molecular weight is 403.45.
Ampicillin Capsules, USP for oral administration provide ampicillin trihydrate equivalent to 250 mg and 500 mg ampicillin. Ampicillin Capsules also contain magnesium stearate. The capsule shell contains black iron oxide, D&C red No. 28, FD&C blue No. 1, gelatin, silicon dioxide, sodium lauryl sulfate, and titanium dioxide.
Ampicillin for Oral Suspension, USP provides ampicillin trihydrate equivalent to 125 mg/5 mL and 250 mg/5 mL ampicillin. Ampicillin for Oral Suspension also contains carboxymethylcellulose sodium, colloidal silicon dioxide, flavors, microcrystalline cellulose, sodium citrate, sodium propionate, and sucrose.
CLINICAL PHARMACOLOGY
Ampicillin is bactericidal at low concentrations and is clinically effective not only against the gram-positive organisms usually susceptible to penicillin G but also against a variety of gram-negative organisms. It is stable in the presence of gastric acid and is well absorbed from the gastrointestinal tract. It diffuses readily into most body tissues and fluids; however, penetration into the cerebrospinal fluid and brain occurs only with meningeal inflammation. Ampicillin is excreted largely unchanged in the urine; its excretion can be delayed by concurrent administration of probenecid which inhibits the renal tubular secretion of ampicillin. In blood serum, ampicillin is the least bound of all the penicillins; an average of about 20 percent of the drug is bound to the plasma proteins as compared to 60 to 90 percent of the other penicillins. The administration of a 500 mg dose of ampicillin trihydrate capsules results in an average peak blood serum level of approximately 3.0 mcg/mL; the average peak serum level for a 250 mg dose of ampicillin trihydrate for oral suspension is approximately 2.3 mcg/mL.
Microbiology:
Mechanism of Action
Ampicillin is similar to penicillin in its bactericidal action against susceptible bacteria during the stage of active multiplication. It acts through the inhibition of cell wall biosynthesis that leads to the death of the bacteria.
Mechanism of Resistance
Resistance to ampicillin is mediated primarily through enzymes called beta-lactamases that cleave the beta-lactam ring of ampicillin, rendering it inactive.
Antimicrobial Activity
Ampicillin has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections, as described in the INDICATIONS AND USAGE section.
| Gram-Positive Bacteria | Gram-Negative Bacteria |
| Enterococcus spp. | Escherichia coli |
| Staphylococcus spp. (non-penicillinase-producing) | Haemophilus influenza (non-penicillinase-producing) |
| Streptococcus pneumoniae | Neisseria gonorrhoeae |
| Streptococcus pyogenes | Neisseria meningitidis |
| Viridans group streptococci | Proteus mirabilis |
| Salmonella spp. |
|
| Shigella spp. |
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to 0.12 mcg/mL for ampicillin. However the efficacy of ampicillin in treating clinical infections due to these bacteria has not been established in adequate and well-controlled trials.
| Gram-Positive Bacteria | Anaerobic Bacteria |
| Bacillus anthracis Corynebacterium xerosis | Clostridium spp. |
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide in vitro susceptibility test results for antimicrobial drugs used in local hospitals and practice areas to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antimicrobial drug product for treatment.
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method1,3 (broth and/or agar). The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion Techniques
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method. 2,3 This procedure uses paper disks impregnated with 10 mcg ampicillin to test the susceptibility of bacteria to ampicillin. The disk diffusion interpretive criteria are provided in Table 1.
Susceptibility to ampicillin of Staphylococcus spp., may be inferred by testing penicillin.3 Perform test(s) to detect beta-lactamase production on staphylococci for which the penicillin MICs are ≤ 0.12 mcg/mL or zone diameters are ≥ 29 mm before reporting isolates as penicillin susceptible. Rare isolates of staphylococci that contain genes for beta-lactamase production may appear negative by beta-lactamase tests. Consequently, for serious infections requiring penicillin therapy, laboratories should perform MIC tests and beta-lactamase testing on all subsequent isolates from the same patient. PCR testing of the isolate for the blaZ beta-lactamase gene may be considered.3
Susceptibility to ampicillin of Streptococcus pneumoniae (non-meningitis isolates), may be inferred by testing penicillin or oxacillin. Non-meningitis isolates with a penicillin MIC ≤0.06 mcg/mL or oxacillin zone size ≥20 mm can be considered susceptible to ampicillin.3
| Minimum Inhibitory Concentrations (mcg/mL) | Disk Diffusion (zone diameters in mm) |
|||||
| Pathogen | S | I | R | S | I | R |
| Enterobacteriaceae | ≤8 | 16 | ≥32 | ≥17 | 14-16 | ≤13 |
| Haemophilus influenzaea | ≤1 | 2 | ≥4 | ≥22 | 19-21 | ≤18 |
| Enterococcusb spp. | ≤8 | - | ≥16 | ≥17 | - | ≤16 |
| Neisseria meningitidis | ≤0.12 | 0.25-1 | ≥2 | - | - | - |
| Viridans group streptococci | ≤0.25 | 0.5-4 | ≥8 | - | - | - |
| Streptococcus pyogenesc | ≤0.25 | - | - | ≥24 | - | - |
| S = Susceptible; I = Intermediate; R = Resistant |
||||||
a. The majority of isolates of H. influenzae that are resistant to ampicillin produce a TEM-type beta-lactamase. In most cases, a direct beta-lactamase test can provide a rapid means of detecting resistance to ampicillin.3
b. Penicillin or ampicillin resistance among enterococci due to beta-lactamase production has been reported very rarely. Penicillin or ampicillin resistance due to beta-lactamase production is not reliably detected with routine disk or dilution methods, but is detected using a direct, nitrocefin-based beta-lactamase test. Because of the rarity of beta-lactamase-positive enterococci, this test need not be performed routinely, but can be used in selected cases. A positive beta-lactamase test predicts resistance to penicillin as well as amino- and ureidopenicillins.3
c. Susceptibility testing of ampicillin need not be performed routinely because nonsusceptible isolates of beta-hemolytic streptococci are extremely rare, and have not been reported for S. pyogenes. If testing is performed, any beta-hemolytic streptococcus isolate that is found to be non-susceptible should be re-identified retested, and if confirmed, reported to the public health laboratory.3
A report of Susceptible indicates that the antimicrobial is likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration usually achievable at the site of infection. A report of Intermediate indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentration usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test. 1,2,3 Standard Ampicillin powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 10 mcg ampicillin disk, the criteria in Table 2 should be achieved.
| QC strain |
Minimum Inhibitory Concentration |
Zone Diameter |
| Escherichia coli ATCC* 25922 | 2 – 8 | 15 – 22 |
| Enterococcus faecalis ATCC 29212 | 0.5 – 2 | - |
| Haemophilus influenzae ATCC 49247 | 2 – 8 | 13 – 21 |
|
Klebsiella pneumoniae ATCC 700603 | >128 | - |
| Staphylococcus aureus ATCC 29213 | 0.5 – 2 | - |
| Staphylococcus aureus ATCC 25923 | - | 27 - 35 |
| Streptococcus pneumoniae ATCC 49619 | 0.06 – 0.25 | 30 – 36 |
| * ATCC = American Type Culture Collection |
||
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ampicillin capsules, Ampicillin for Oral Suspension and other antibacterial drugs, Ampicillin capsules and Ampicillin for Oral Suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. When culture and susceptibility information are available, they should be considered in selecting of modifying antimicrobial therapy, in the absence of such data, local epidemiology and susceptibility patterns contribute to the empiric selection of therapy.
Ampicillin capsules and Ampicillin for oral suspension are indicated in the treatment of infections caused by susceptible strains of the designated organisms listed below:
Infections of the genitourinary tract including gonorrhea - E. coli, P. mirabilis, enterococci, Shigella, S. typhosa and other Salmonella and nonpenicillinase-producing N. gonorrhoeae.
Infections of the respiratory tract - Nonpenicillinase-producing H. influenzae and staphylococci, and streptococci including Streptococcus pneumoniae.
Infections of the gastrointestinal tract - Shigella, S. typhosa and other Salmonella, E. coli, P. mirabilis, and enterococci.
Meningitis - N. Meningitidis
Bacteriology studies to determine the causative organisms and their susceptibility to ampicillin should be performed. Therapy may be instituted prior to the results of susceptibility testing.
CONTRAINDICATIONS
A history of a previous hypersensitivity reaction to any of the penicillins is a contraindication. Ampicillin is also contraindicated in infections caused by penicillinase-producing organisms.
WARNINGS
SERIOUS AND OCCASIONALLY FATAL HYPERSENSITIVITY (ANAPHYLACTIC) REACTIONS HAVE BEEN REPORTED IN PATIENTS ON PENICILLIN THERAPY. ALTHOUGH ANAPHYLAXIS IS MORE FREQUENT FOLLOWING PARENTERAL THERAPY, IT HAS OCCURRED IN PATIENTS ON ORAL PENICILLINS. THESE REACTIONS ARE MORE LIKELY TO OCCUR IN INDIVIDUALS WITH A HISTORY OF SENSITIVITY TO MULTIPLE ALLERGENS. THERE HAVE BEEN REPORTS OF INDIVIDUALS WITH A HISTORY OF PENICILLIN HYPERSENSITIVITY WHO HAVE EXPERIENCED SEVERE REACTIONS WHEN TREATED WITH CEPHALOSPORINS. BEFORE THERAPY WITH ANY PENICILLIN, CAREFUL INQUIRY SHOULD BE MADE CONCERNING PREVIOUS HYPERSENSITIVITY REACTIONS TO PENICILLINS, CEPHALOSPORINS, OR OTHER ALLERGENS. IF AN ALLERGIC REACTION OCCURS, APPROPRIATE THERAPY SHOULD BE CONSIDERED. SERIOUS ANAPHYLACTIC REACTIONS REQUIRE IMMEDIATE EMERGENCY TREATMENT WITH EPINEPHRINE. OXYGEN, INTRAVENOUS STEROIDS, AND AIRWAY MANAGEMENT, INCLUDING INTUBATION, SHOULD ALSO BE ADMINISTERED AS INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including ampicillin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated colitis".
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
PRECAUTIONS
General
Prescribing Ampicillin capsules and Ampicillin for Oral Suspension in the absence of a proven or strongly suspected bacterial infection of a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Prolonged use of antibiotics may promote the overgrowth of nonsusceptible organisms, including fungi. Should superinfection occur, appropriate measures should be taken.
Patients with gonorrhea who also have syphilis should be given additional appropriate parenteral penicillin treatment.
Treatment with ampicillin does not preclude the need for surgical procedures, particularly in staphylococcal infections.
Information for the patient
1. The patient should inform the physician of any history of sensitivity to allergens, including previous hypersensitivity reactions to penicillins and cephalosporins (see WARNINGS).
2. The patient should discontinue ampicillin and contact the physician immediately if any side effect occurs (see WARNINGS).
3. Ampicillin should be taken with a full glass (8 oz) of water, one-half hour before or two hours after meals.
4. Diabetic patients should consult with the physician before changing diet or dosage of diabetes medication (see PRECAUTIONS, Drug/Laboratory Test Interactions).
Patients should be counseled that antibacterial drugs, including Ampicillin capsules and Ampicillin for Oral Suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g. the common cold).
When Ampicillin capsules or Ampicillin for Oral Suspension are prescribed to treat a bacterial infection, patients should be told that, although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may:
(i) Decrease the effectiveness of the immediate treatment, and
(ii) Increase the likelihood that bacteria will develop resistance and will not be treatable by Ampicillin capsules and Ampicillin for Oral Suspension or other antibacterial drugs in the future
Laboratory Tests
In prolonged therapy, and particularly with high dosage regimens, periodic evaluation of the renal, hepatic and hematopoietic systems is recommended. In streptococcal infections, therapy must be sufficient to eliminate the organism (10 days minimum); otherwise the sequelae of streptococcal disease may occur. Cultures should be taken following completion of treatment to determine whether streptococci have been eradicated. Cases of gonococcal infection with a suspected lesion of syphilis should have darkfield examinations ruling out syphilis before receiving ampicillin. Patients who do not have suspected lesions of syphilis and are treated with ampicillin should have a follow-up serologic test for syphilis each month for four months to detect syphilis that may have been masked from treatment for gonorrhea.
Drug Interactions
When administered concurrently, the following drugs may interact with ampicillin:
Allopurinol - Increased possibility of skin rash; particularly in hyperuricemic patients may occur.
Bacteriostatic antibiotics - Chloramphenicol, erythromycins, sulfonamides, or tetracyclines may interfere with the bactericidal effect of penicillins. This has been demonstrated in vitro; however, the clinical significance of this interaction is not well-documented.
Oral contraceptives - May be less effective and increased breakthrough bleeding may occur.
Probenecid - May decrease renal tubular secretion of ampicillin resulting in increased blood levels and/or ampicillin toxicity.
Drug/Laboratory Test Interactions
After treatment with ampicillin, a false-positive reaction for glucose in the urine may occur with copper sulfate tests (Benedict's solution, Fehling's solution, or Clinitest® tablets) but not with enzyme based tests such as Clinistix® and Tes-Tape® (Glucose Enzymatic Test Strip, USP).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenesis, mutagenesis, or impairment of fertility in males or females.
Pregnancy
Teratogenic Effects
Category B:
Reproduction studies in animals have revealed no evidence of impaired fertility or harm to the fetus due to penicillin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, penicillin should be used during pregnancy only if clearly needed.
Labor and Delivery
Oral ampicillin-class antibiotics are poorly absorbed during labor. Studies in guinea pigs showed that intravenous administration of ampicillin slightly decreased the uterine tone and frequency of contractions, but moderately increased the height and duration of contractions. However, it is not known whether use of these drugs in humans during labor or delivery has immediate or delayed adverse effects on the fetus, prolongs the duration of labor, or increases the likelihood that forceps delivery or other obstetrical intervention or resuscitation of the newborn will be necessary.
Nursing Mothers
Ampicillin-class antibiotics are excreted in milk. Ampicillin used by nursing mothers may lead to sensitization of infants; therefore, a decision should be made whether to discontinue nursing or to discontinue ampicillin, taking into account the importance of the drug to the mother.
Pediatric use
Penicillins are excreted primarily unchanged by the kidney; therefore, the incompletely developed renal function in neonates and young infants will delay the excretion of penicillin. Administration to neonates and young infants should be limited to the lowest dosage compatible with an effective therapeutic regime (see DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
As with other penicillins, it may be expected that untoward reactions will be essentially limited to sensitivity phenomena. They are more likely to occur in individuals who have previously demonstrated hypersensitivity to penicillin and in those with a history of allergy, asthma, hay fever or urticaria.
The following adverse reactions have been reported as associated with the use of ampicillin:
Gastrointestinal: glossitis, stomatitis, nausea, vomiting, enterocolitis, pseudomembranous colitis, and diarrhea. These reactions are usually associated with oral dosage forms of the drug.
Hypersensitivity Reactions: An erythematous, mildly pruritic, maculopapular skin rash has been reported fairly frequently. The rash, which usually does not develop within the first week of therapy, may cover the entire body including the soles, palms, and oral mucosa. The eruption usually disappears in three to seven days. Other hypersensitivity reactions that have been reported are: skin rash, pruritus, urticaria, erythema multiforme, and an occasional case of exfoliative dermatitis. Anaphylaxis is the most serious reaction experienced and has usually been associated with the parenteral dosage form of the drug.
NOTE: Urticaria, other skin rashes, and serum sickness-like reactions may be controlled by antihistamines, and, if necessary, systemic corticosteroids. Whenever such reactions occur, ampicillin should be discontinued unless, in the opinion of the physician, the condition being treated is life-threatening and amenable only to ampicillin therapy. Serious anaphylactic reactions require emergency measures (see WARNINGS).
Liver: Moderate elevation in serum glutamic oxaloacetic transaminase (SGOT) has been noted, but the significance of this finding is unknown.
Hemic and Lymphatic Systems: anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis have been reported during therapy with penicillins. These reactions are usually reversible on discontinuation of therapy and are believed to be hypersensitivity phenomena.
Other adverse reactions that have been reported with the use of ampicillin are laryngeal stridor and high fever. An occasional patient may complain of sore mouth or tongue as with any oral penicillin preparation.
OVERDOSAGE
In case of overdosage, discontinue medication, treat symptomatically and institute supportive measures as required. In patients with renal function impairment, ampicillin-class antibiotics can be removed by hemodialysis but not by peritoneal dialysis.
DOSAGE AND ADMINISTRATION
Adults and children weighing over 20 Kg: For genitourinary or gastrointestinal tract infections other than gonorrhea in men and women, the usual dose is 500 mg q.i.d. in equally spaced doses; severe or chronic infections may require larger doses. For the treatment of gonorrhea in both men and women, a single oral dose of 3.5 grams of ampicillin administered simultaneously with 1 gram of probenecid is recommended. Physicians are cautioned to use no less than the above recommended dosage for the treatment of gonorrhea. Follow-up cultures should be obtained from the original site(s) of infection 7 to 14 days after therapy. In women, it is also desirable to obtain culture test-of-cure from both the endocervical and anal canals. Prolonged intensive therapy is needed for complications such as prostatitis and epididymitis. For respiratory tract infections, the usual dose is 250 mg q.i.d. in equally spaced doses.
Pediatric Patients weighing 20 Kg or less: For genitourinary or gastrointestinal tract infections, the usual dose is 100 mg/kg/day total, q.i.d. in equally divided and spaced doses.
For respiratory tract infections, the usual dose is 50 mg/kg/day total, in equally divided and spaced doses three to four times daily. Doses for children should not exceed doses recommended for adults.
All patients, irrespective of age and weight: Larger doses may be required for severe or chronic infections. Although ampicillin is resistant to degradation by gastric acid, it should be administered at least one half-hour before or two hours after meals for maximal absorption. Except for the single dose regimen for gonorrhea referred to above, therapy should be continued for a minimum of 48 to 72 hours after the patient becomes asymptomatic or evidence that bacterial eradication has been obtained. In infections caused by hemolytic strains of streptococci, a minimum of 10 days' treatment is recommended to guard against the risk of rheumatic fever or glomerulonephritis (see PRECAUTIONS, Laboratory Tests). In the treatment of chronic urinary or gastrointestinal infections, frequent bacteriologic and clinical appraisal is necessary during therapy and may be necessary for several months afterwards. Stubborn infections may require treatment for several weeks. Smaller doses than those indicated above should not be used.
Directions for mixing Oral Suspension
Prepare suspension at time of dispensing. For ease of preparation, add water to the bottle in two portions and shake well after each addition.
125 mg/5 mL
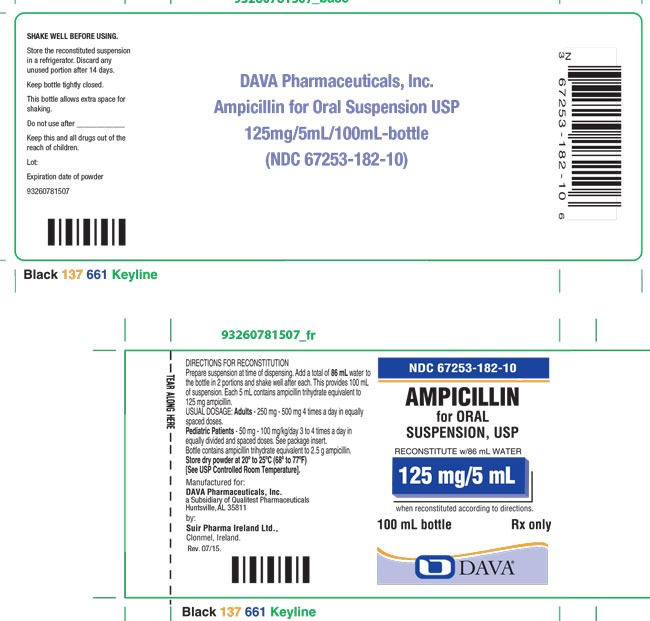
Add a total of 86 mL to the 100 mL package and 170 mL to the 200 mL package. This will provide 100 mL and 200 mL of suspension, respectively. Each 5 mL (teaspoonful) will contain ampicillin trihydrate equivalent to 125 mg ampicillin.
250 mg/5 mL
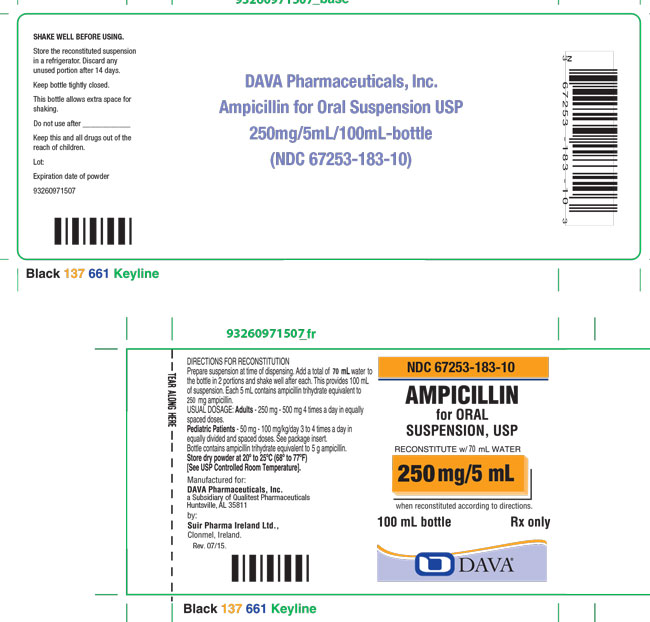
Add a total of 70 mL to the 100 mL package and 139 mL to the 200 mL package. This will provide 100 mL and 200 mL of suspension, respectively. Each 5 mL (teaspoonful) will contain ampicillin trihydrate equivalent to 250 mg ampicillin.
REFERENCES
1. Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Tenth Edition. CLSI document M07-A10, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
2. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standard – Twelfth Edition. CLSI document M02-A12, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
3. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-fifth Informational Supplement, CLSI document M100-S25. CLSI document M100-S25, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
HOW SUPPLIED
Ampicillin Capsules, USP 250 mg: Each capsule contains ampicillin trihydrate equivalent to 250 mg ampicillin. The number 2 size capsule has a gray opaque body with a light blue opaque cap, printed WC402.
Bottles of 100 NDC 67253-180-10
Bottles of 500 NDC 67253-180-50
Ampicillin Capsules, USP 500 mg: Each capsule contains ampicillin trihydrate equivalent to 500 mg ampicillin. The number 0 size capsule has a gray opaque body with a light blue opaque cap, printed WC404.
Bottles of 100 NDC 67253-181-10
Bottles of 500 NDC 67253-181-50
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Ampicillin for Oral Suspension, USP is available as a powder which when reconstituted as directed yields a white, bubble gum flavored suspension.
Ampicillin for Oral Suspension, USP 125 mg/5 mL: Each 5 mL of reconstituted suspension contains ampicillin trihydrate equivalent to 125 mg ampicillin.
100 mL bottles NDC 67253-182-10
200 mL bottles NDC 67253-182-20
Ampicillin for Oral Suspension, USP 250 mg/5 mL: Each 5 mL of reconstituted suspension contains ampicillin trihydrate equivalent to 250 mg ampicillin.
100 mL bottles NDC 67253-183-10
200 mL bottles NDC 67253-183-20
Store dry powder at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Store the reconstituted suspension in a refrigerator. Discard any unused portion after 14 days.
Manufactured for:
DAVA Pharmaceuticals, Inc.
a Subsidiary of Qualitest Pharmaceuticals
Huntsville, AL 35811
Manufactured by:
Suir Pharma Ireland Ltd.
Clonmel, Ireland.
Rev. 07/15
| AMPICILLIN
ampicillin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| AMPICILLIN
ampicillin capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| AMPICILLIN
ampicillin suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| AMPICILLIN
ampicillin suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Par Pharmaceutical (172202025) |