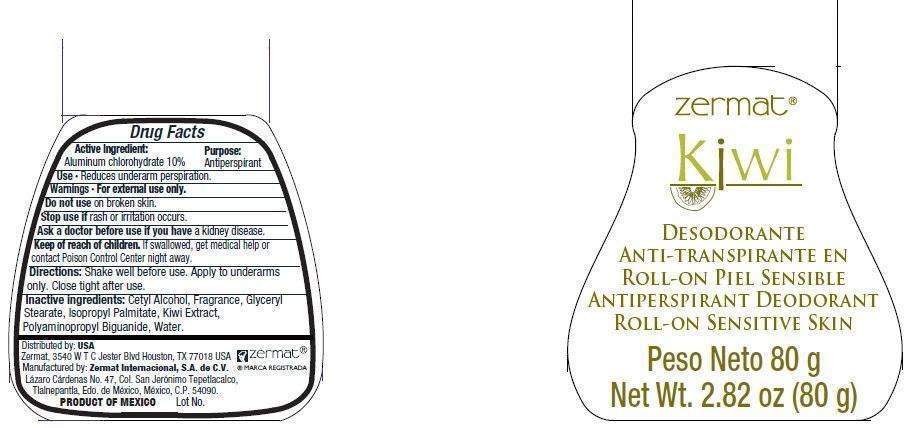
Label: ZERMAT KIWI ANTIPERSPIRANT DEODORANT ROLL-ON SENSITIVE SKIN- aluminum chlorohydrate liquid
- NDC Code(s): 15828-880-01
- Packager: Zermat Internacional
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- zermat Kiwi ANTIPERSPIRANT DEODORANT ROLL-ON SENSITIVE SKIN
- Active Ingredient
- Use
- Warnings
- DO NOT USE
- STOP USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive Ingredients
- zermat Kiwi ANTIPERSPIRANT DEODORANT ROLL-ON SENSITIVE SKIN 80g (15828-880-01)
-
INGREDIENTS AND APPEARANCE
ZERMAT KIWI ANTIPERSPIRANT DEODORANT ROLL-ON SENSITIVE SKIN
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15828-880 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 10 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) KIWI FRUIT (UNII: 71ES77LGJC) POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15828-880-01 80 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 10/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 10/02/2013 Labeler - Zermat Internacional (812820712) Registrant - Zermat Internacional (812820712) Establishment Name Address ID/FEI Business Operations Zermat Internacional 812820712 manufacture(15828-880)