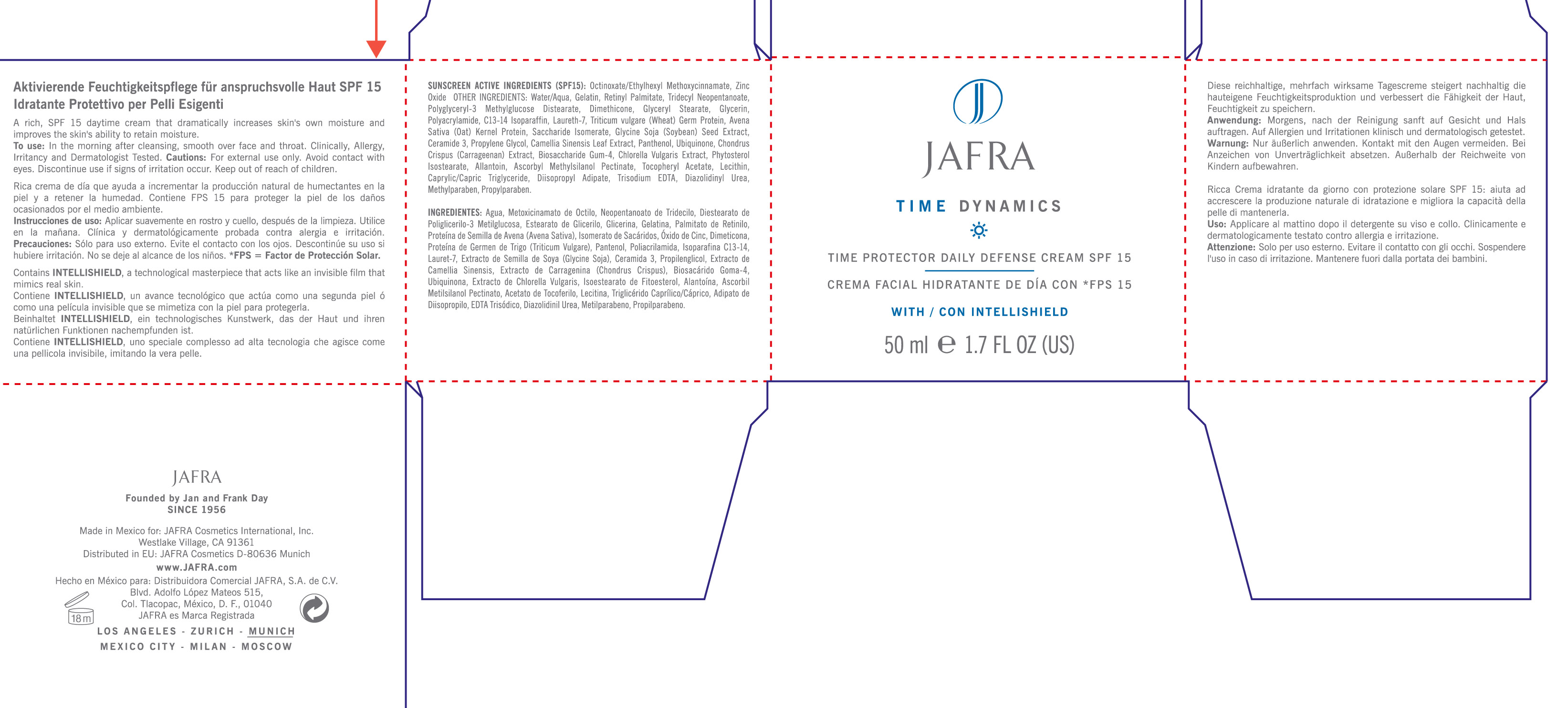
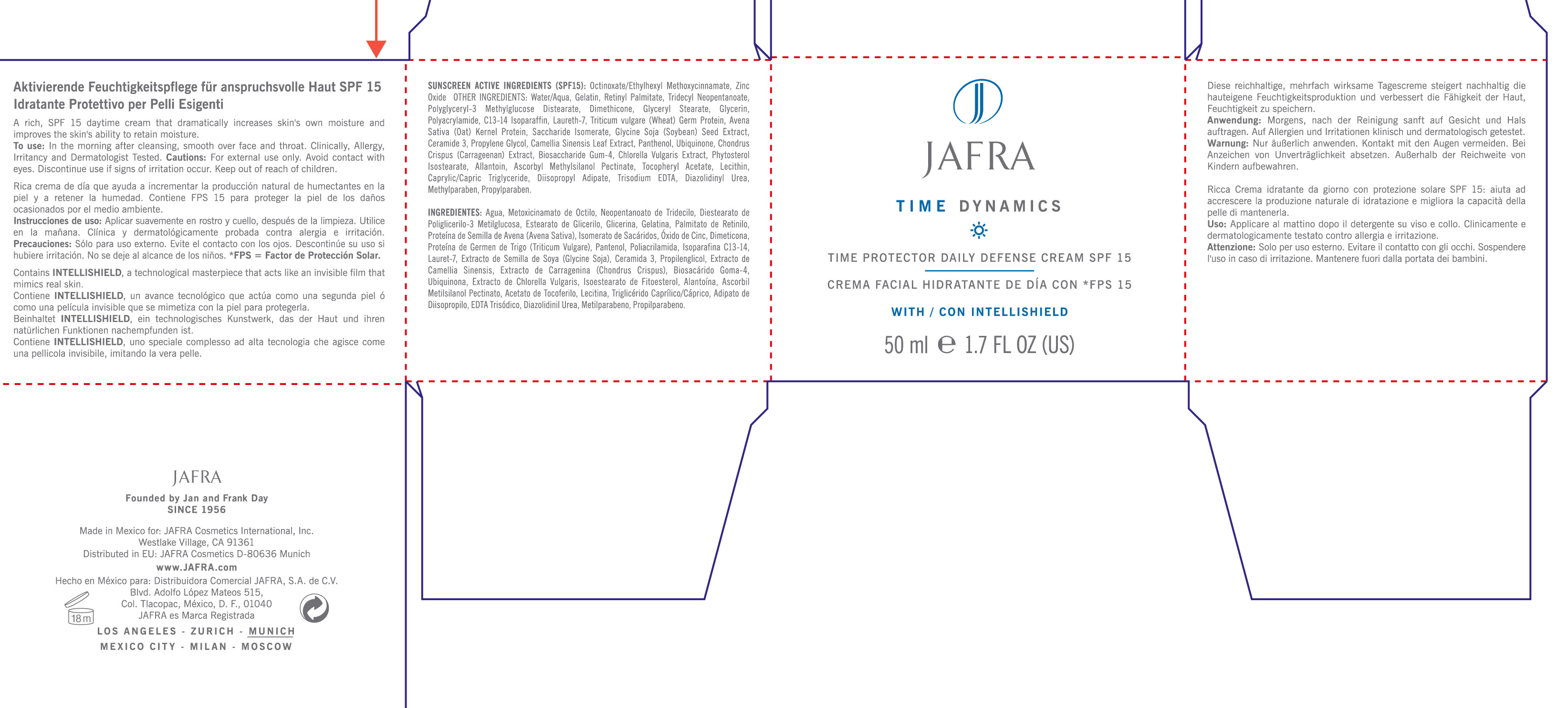
Label: TIME DYNAMICS TIME PROTECTOR DAILY DEFENSE SPF 15- octinoxate, zinc oxide cream, augmented
-
Contains inactivated NDC Code(s)
NDC Code(s): 68828-098-01 - Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 13, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Sunscreen Active Ingredients (SPF 15): Octinoxate/Ethylhexyl Methoxycinnamate, Zinc Oxide
To use: In the morning after cleansing. Smooth over face and throat. Clinically, allergy, irritancy tested.
Other ingredients: Water/Aqua, Gelatin, Retinyl Palmitate, Tridecyl Neopentanoate, Polyglyceryl-3 Methylglucose Distearate, Dimethicone, Glyceryl Stearate, Glycerin, Polacrylamide, C13-14 Isoparaffin, Laureth-7, Triticum Vulgare (wheat) Germ Protein, Avena Sativa (Oat) Kernel Protein, Saccharide Isomerate, Glycine Soja (Soybean) Seed Extract, Ceramide 3, Propylene Glycol, Camellia Sinensis Leaf Extract, Panthenol, Ubiquinone, Chondrus Crispus (Carrageenan) Extract, Biosaccharide Gum-4, Chlorella Vulgaris Extract, Phytosterol Isostearate, Allantoin, Ascorbyl Methylsilanol Pectinate, Tocopheryl Acetate, Lecithin, Caprylic/Capric Triglyceride, Diisopropyl Adipate, Trisodium EDTA, Diazolidinyl Urea, Methylparaben, Propylparaben.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TIME DYNAMICS TIME PROTECTOR DAILY DEFENSE SPF 15
octinoxate, zinc oxide cream, augmentedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-098 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.75 mL in 50 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.5 mL in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GELATIN (UNII: 2G86QN327L) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) TRIDECYL NEOPENTANOATE (UNII: 3Z8H1DA7J5) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) LAURETH-7 (UNII: Z95S6G8201) WHEAT GERM (UNII: YR3G369F5A) SACCHARIDE ISOMERATE (UNII: W8K377W98I) SOYBEAN (UNII: L7HT8F1ZOD) CERAMIDE 3 (UNII: 4370DF050B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PANTHENOL (UNII: WV9CM0O67Z) UBIDECARENONE (UNII: EJ27X76M46) CHONDRUS CRISPUS (UNII: OQS23HUA1X) CHLORELLA VULGARIS (UNII: RYQ4R60M02) ALLANTOIN (UNII: 344S277G0Z) ASCORBYL METHYLSILANOL PECTINATE (UNII: M3PF9237YC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE TRISODIUM (UNII: 420IP921MB) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-098-01 1 in 1 CARTON 1 50 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/30/2012 Labeler - Jafra Cosmetics International Inc (041676479) Registrant - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Jafra Manufacturing, S.A. de C.V. 814732061 manufacture