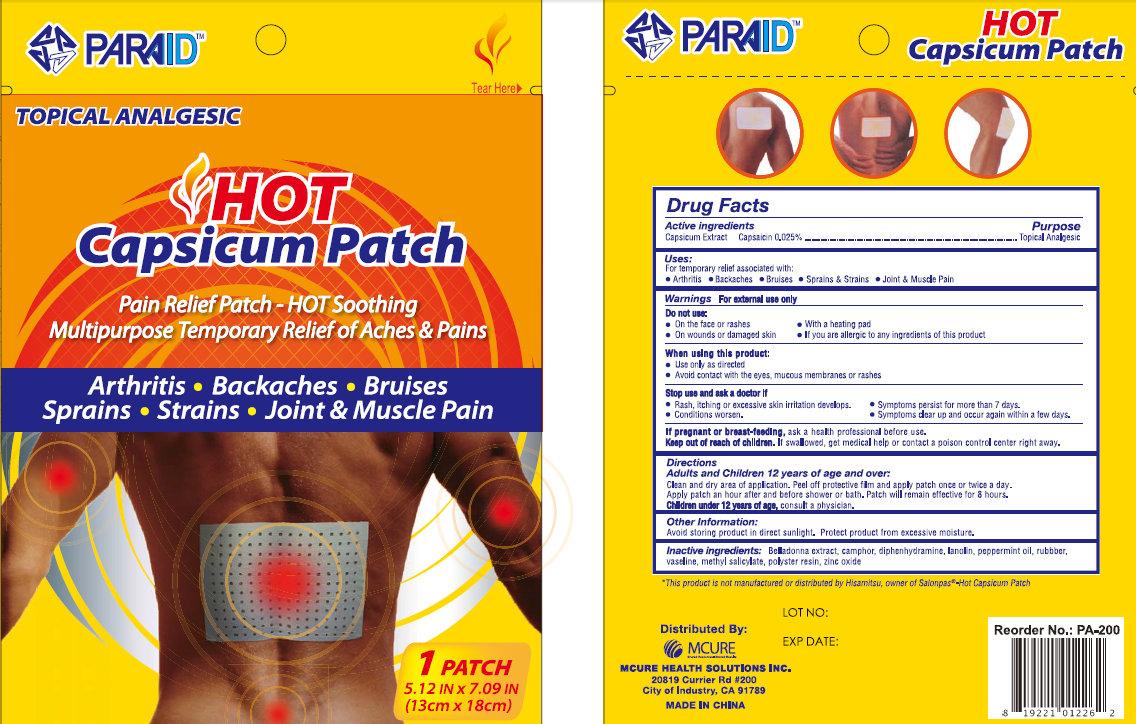
PARAID HOT CAPSICUM- capsaicin patch
Mcure Health Solutions Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PARAID Hot Capsicum Patch
Uses:
For temporary relief associated with:
• Arthritis • Backaches • Bruises • Sprains
& Strains • Joint & Muscle Pain
Do not use:
• On the face or rashes • With a heating pad
• On wounds or damaged skin • If you are allergic to any ingredients of this product
When using this product:
• Use only as directed
• Avoid contact with the eyes, mucous membranes or rashes
Stop use and ask a doctor if
• Rash, itching or excessive skin irritation develops. • Symptoms persist for more than 7 days.
• Conditions worsen. • Symptoms clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and Children 12 years of age and over:
Clean and dry area of application. Peel off protective film and apply patch once or twice a day.
Apply patch an hour after and before shower or bath. Patch will remain effective for 8 hours.
Children under 12 years of age, consult a physician.
Inactive Ingredients:
Belladonna extract, camphor, diphenhydramine, lanolin, peppermint oil, rubbber, vaseline, methyl salicylate, polyster resin, zinc oxide
| PARAID HOT CAPSICUM
capsaicin patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mcure Health Solutions Inc. (053034873) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Dingtai Pharmaceutical Co., Ltd | 420598724 | manufacture(52486-022) | |