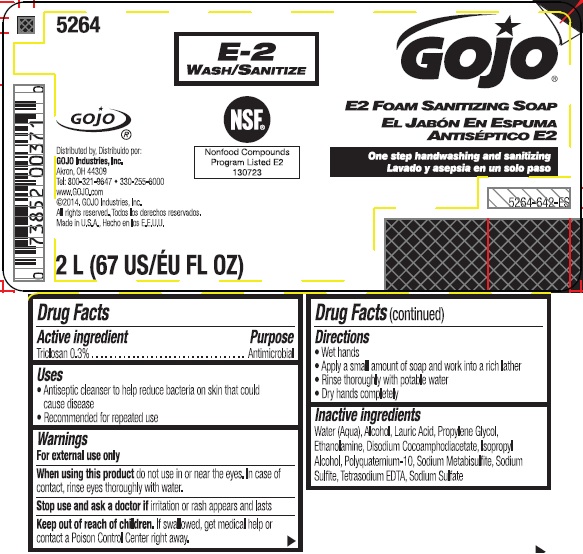
GOJO E2 FOAM SANITIZING SO AP- triclosan liquid
GOJO Industries, Inc.
----------
GOJO E2 Foam Sanitizing Soap
Use
Antiseptic cleanser to help reduce bacteria on skin that could cause disease
Recommended for repeated use
Warnings
For external use only
Directions
- Wet hands
- Apply a small amount of soap and work into a rich lather
- Rinse thoroughly with potable water
- Dry hands completely
| GOJO E2 FOAM SANITIZING SO AP
triclosan liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - GOJO Industries, Inc. (004162038) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GOJO Industries, Inc. | 036424534 | manufacture(21749-543) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GOJO Industries, Inc. | 088312414 | label(21749-543) , pack(21749-543) | |
Revised: 3/2024
Document Id: 6b64b777-1377-4e5f-903c-00970a2be5fd
Set id: 11483a33-1bb9-4c7f-8d16-b84064d4eec3
Version: 2
Effective Time: 20240321
GOJO Industries, Inc.