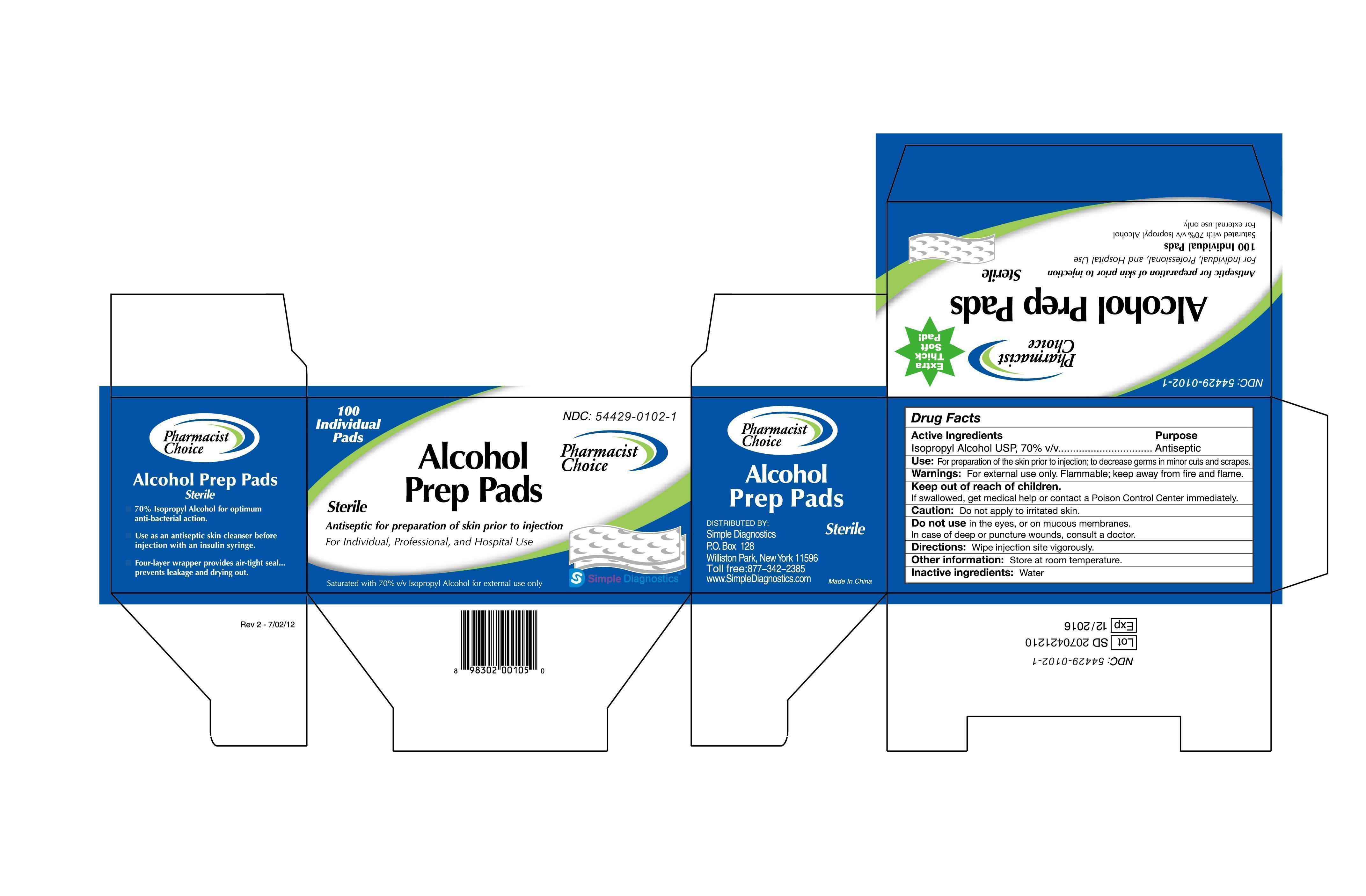
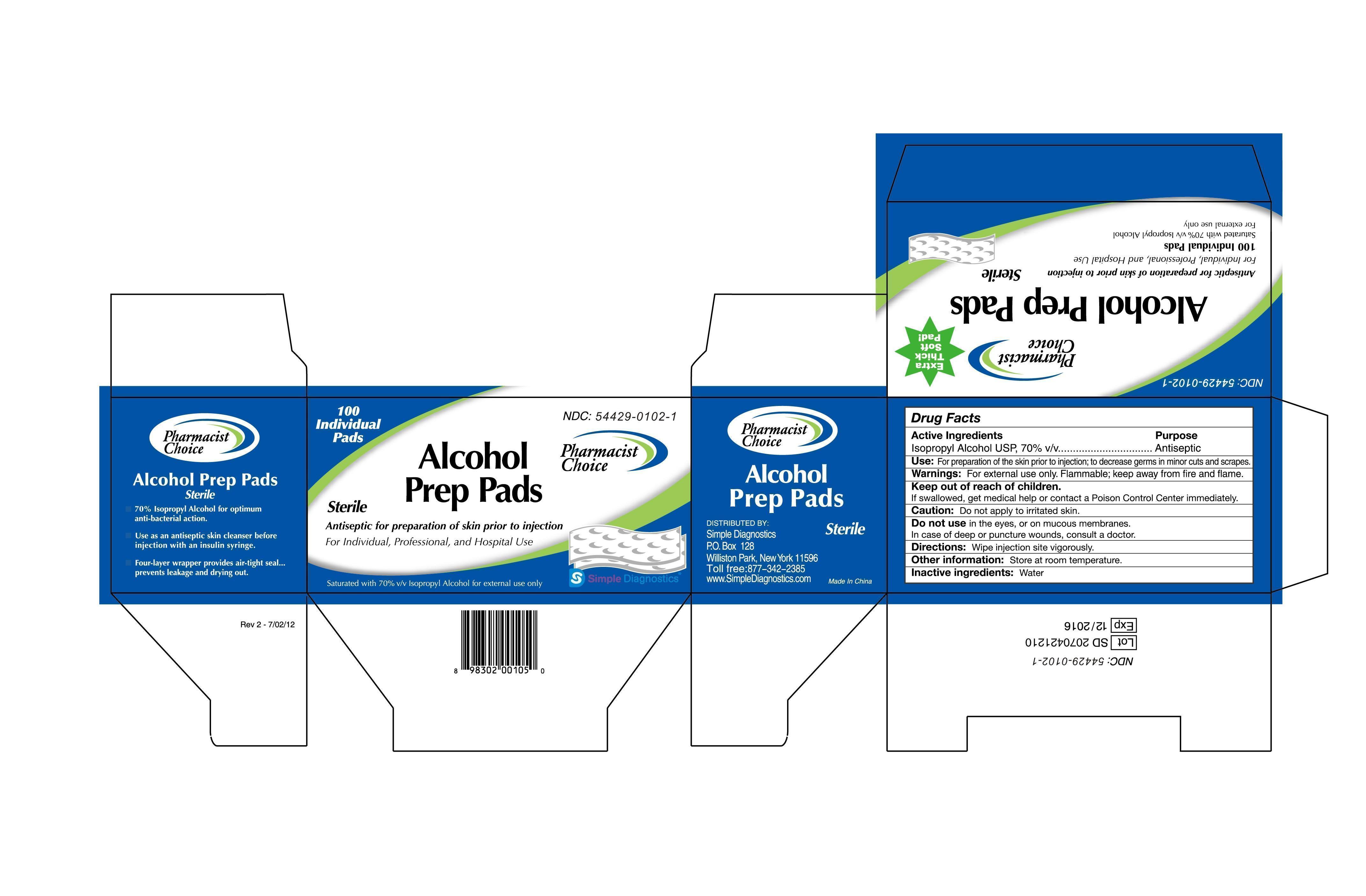
Label: PHARMACIST CHOICE ALCOHOL STERILE PREP PAD- isopropyl alcohol swab
- NDC Code(s): 62379-005-05
- Packager: Simple Diagnostics
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep out of children
- Use
- Warning
- Directions
- Inactive ingredients
-
Drug Facts
Active Ingredients
Isopropyl Alcohol USP, 70% v/v
Purpose:
Antiseptic
Use: For preparation of skin prior to injection; to decrease germs in minor cuts and scrapes.
Warning: For external use only. Flammable, keep away from fire and flame.
Keep out of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Caution: Don’t apply to a irritated skin.
Don’t use in the eyes, or on mucous membranes.
In case of deep or puncture wounds, consult a doctor.
Directions: wipe injection site vigorously.
Other information: Store at room temperature.
Inactive ingredients: Water
-
INGREDIENTS AND APPEARANCE
PHARMACIST CHOICE ALCOHOL STERILE PREP PAD
isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62379-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62379-005-05 1.4 mL in 1 POUCH; Type 0: Not a Combination Product 10/10/2017 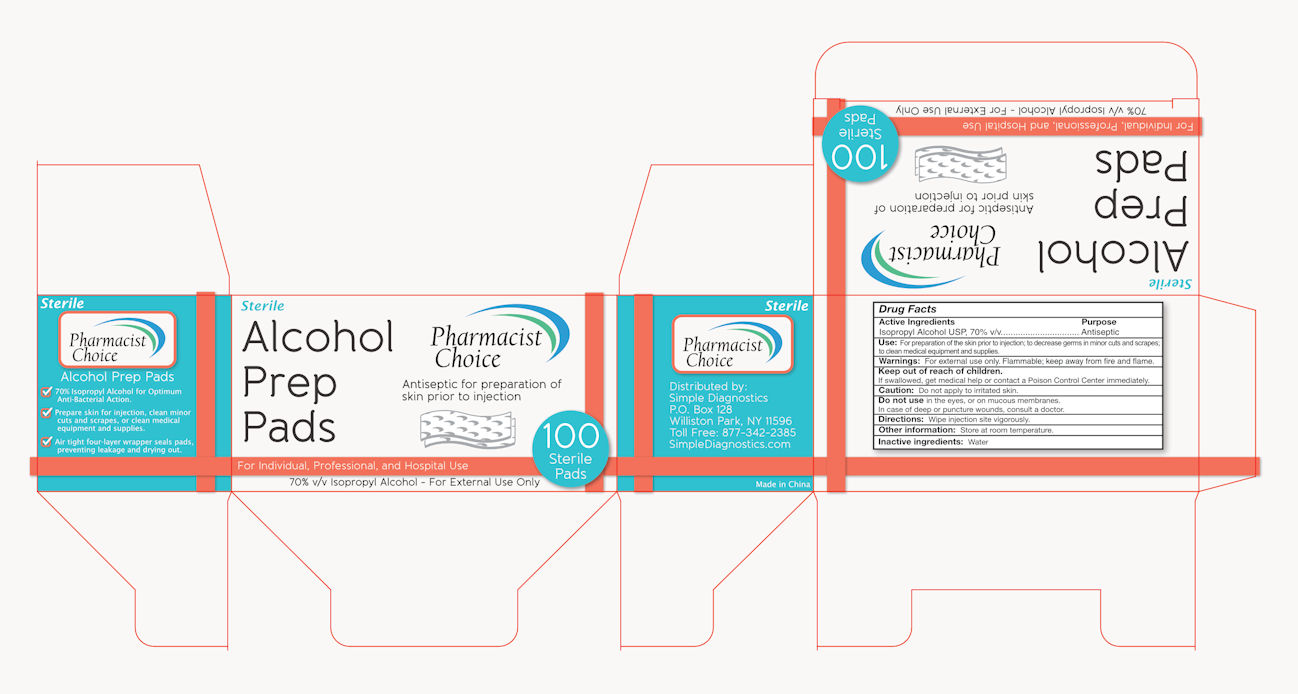
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 02/23/2015 Labeler - Simple Diagnostics (004135503) Registrant - Simple Diagnostics (004135503)