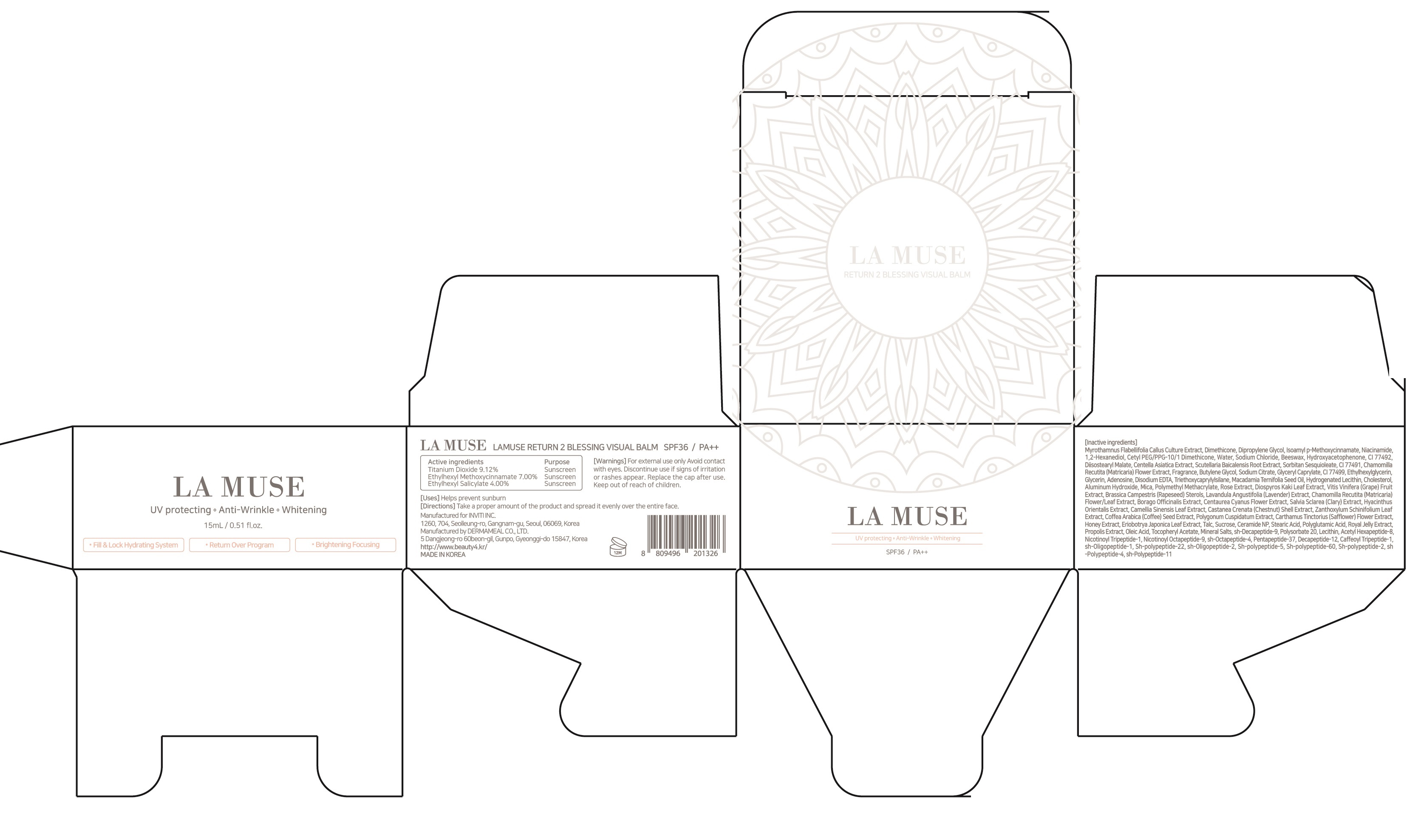
Label: LAMUSE RETURN 2 BLESSING VISUAL BALM- titanium dioxide, octinoxate, octisalate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72161-010-01, 72161-010-02 - Packager: INVITI INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 15, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredients:
Inactive ingredients: Myrothamnus Flabellifolia Callus Culture Extract, Dimethicone, Dipropylene Glycol, Isoamyl p-Methoxycinnamate, Niacinamide, 1,2-Hexanediol, Cetyl PEG/PPG-10/1 Dimethicone, Water, Sodium Chloride, Beeswax, Hydroxyacetophenone, CI 77492, Diisostearyl Malate, Centella Asiatica Extract, Scutellaria Baicalensis Root Extract, Sorbitan Sesquioleate, CI 77491, Chamomilla Recutita (Matricaria) Flower Extract, Fragrance, Butylene Glycol, Sodium Citrate, Glyceryl Caprylate, CI 77499, Ethylhexylglycerin, Glycerin, Adenosine, Disodium EDTA, Triethoxycaprylylsilane, Macadamia Ternifolia Seed Oil, Hydrogenated Lecithin, Cholesterol, Aluminum Hydroxide, Mica, Polymethyl Methacrylate, Rose Extract, Diospyros Kaki Leaf Extract, Vitis Vinifera (Grape) Fruit Extract, Brassica Campestris (Rapeseed) Sterols, Lavandula Angustifolia (Lavender) Extract, Chamomilla Recutita (Matricaria) Flower/Leaf Extract, Borago Officinalis Extract, Centaurea Cyanus Flower Extract, Salvia Sclarea (Clary) Extract, Hyacinthus Orientalis Extract, Camellia Sinensis Leaf Extract, Castanea Crenata (Chestnut) Shell Extract, Zanthoxylum Schinifolium Leaf Extract, Coffea Arabica (Coffee) Seed Extract, Polygonum Cuspidatum Extract, Carthamus Tinctorius (Safflower) Flower Extract, Honey Extract, Eriobotrya Japonica Leaf Extract, Talc, Sucrose, Ceramide NP, Stearic Acid, Polyglutamic Acid, Royal Jelly Extract, Propolis Extract, Oleic Acid, Tocopheryl Acetate, Mineral Salts, sh-Decapeptide-9, Polysorbate 20, Lecithin, Acetyl Hexapeptide-8, Nicotinoyl Tripeptide-1, Nicotinoyl Octapeptide-9, sh-Octapeptide-4, Pentapeptide-37, Decapeptide-12, Caffeoyl Tripeptide-1, sh-Oligopeptide-1, Sh-polypeptide-22, sh-Oligopeptide-2, Sh-polypeptide-5, Sh-polypeptide-60, Sh-polypeptide-2, sh-Polypeptide-4, sh-Polypeptide-11 - PURPOSE
- WARNINGS
- DESCRIPTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LAMUSE RETURN 2 BLESSING VISUAL BALM
titanium dioxide, octinoxate, octisalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72161-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium Dioxide 1.36 g in 15 mL Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 1.05 g in 15 mL Octisalate (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) Octisalate 0.60 g in 15 mL Inactive Ingredients Ingredient Name Strength Dimethicone (UNII: 92RU3N3Y1O) Dipropylene Glycol (UNII: E107L85C40) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72161-010-02 1 in 1 CARTON 02/01/2018 1 NDC:72161-010-01 15 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2018 Labeler - INVITI INC. (694812156) Registrant - INVITI INC. (694812156) Establishment Name Address ID/FEI Business Operations DERMAMEAL CO., LTD. 694253327 manufacture(72161-010)