PHENYLEPHRINE HYDROCHLORIDE- phenylephrine hydrochloride injection
Cipla USA Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PHENYLEPHRINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for PHENYLEPHRINE HYDROCHLORIDE INJECTION.
PHENYLEPHRINE HYDROCHLORIDE injection, for intravenous use Initial U.S. Approval:2012 INDICATIONS AND USAGEPhenylephrine hydrochloride is an alpha-1 adrenergic receptor agonist indicated for increasing blood pressure in adults with clinically important hypotension resulting primarily from vasodilation, in such settings as septic shock or anesthesia. (1) DOSAGE AND ADMINISTRATIONDilute before administration. (2.1) Dosing for Perioperative Hypotension
Dosing for Patients with Vasodilatory Shock
CONTRAINDICATIONSHypersensitivity to it or any of its components (4) WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse reactions: nausea and vomiting, headache, nervousness (6) To report SUSPECTED ADVERSE REACTIONS, contact Cipla Ltd at 1-866-604-3268 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 12/2018 |
FULL PRESCRIBING INFORMATION
2 DOSAGE AND ADMINISTRATION
2.1 General Administration Instructions
Phenylephrine hydrochloride injection must be diluted before administration as bolus intravenous infusion or continuous intravenous infusion.
Inspect the solution for particulate matter and discoloration prior to administration. The diluted solution should not be held for more than 4 hours at room temperature or for more than 24 hours under refrigerated conditions. Discard any unused portion.
During phenylephrine hydrochloride injection administration:
- Correct intravascular volume depletion.
- Correct acidosis. Acidosis may reduce the effectiveness of phenylephrine.
2.2 Preparing a 100 mcg/mL Solution for Bolus Intravenous Administration
For bolus intravenous administration, withdraw 10 mg (1 mL of a 10 mg/mL concentration) of phenylephrine hydrochloride injection and dilute with 99 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP. This will yield a final concentration of 100 mcg/mL. Withdraw an appropriate dose from the 100 mcg/mL solution prior to bolus intravenous administration.
2.3 Preparing a Solution for Continuous Intravenous Infusion
For continuous intravenous infusion, withdraw 10 mg (1 mL of 10 mg/mL concentration) of phenylephrine hydrochloride injection and add to 500 mL of 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP (providing a final concentration of 20 mcg/mL).
2.4 Dosing for Perioperative Setting
In adult patients undergoing surgical procedures with either neuraxial anesthesia or general anesthesia:
- 50 mcg to 250 mcg by intravenous bolus administration. The most frequently reported initial bolus dose is 50 mcg or 100 mcg.
- 0.5 mcg/kg/min to 1.4 mcg/kg/min by intravenous continuous infusion, titrated to blood pressure goal.
2.5 Dosing for Septic or Other Vasodilatory Shock
In adult patients with septic or other vasodilatory shock:
- No bolus.
- 0.5 mcg/kg/min to 6 mcg/kg/min by intravenous continuous infusion, titrated to blood pressure goal. Doses above 6 mcg/kg/min do not show significant incremental increase in blood pressure.
5 WARNINGS AND PRECAUTIONS
5.1 Exacerbation of Angina, Heart Failure, or Pulmonary Arterial Hypertension
Because of its pressor effects, phenylephrine hydrochloride can precipitate angina in patients with severe arteriosclerosis or history of angina, exacerbate underlying heart failure, and increase pulmonary arterial pressure.
5.2 Bradycardia
Phenylephrine hydrochloride can cause severe bradycardia and decreased cardiac output.
5.3 Risk in Patients with Autonomic Dysfunction
The pressor response to adrenergic drugs, including phenylephrine, can be increased in patients with autonomic dysfunction, as may occur with spinal cord injuries.
5.4 Skin and Subcutaneous Necrosis
Extravasation of phenylephrine can cause necrosis or sloughing of tissue.
5.5 Pressor Effect with Concomitant Oxytocic Drugs
Oxytocic drugs potentiate the pressor effect of sympathomimetic pressor amines including phenylephrine hydrochloride [see Drug Interactions (7.1)], with the potential for hemorrhagic stroke.
5.6 Allergic Reactions
This product contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
6 ADVERSE REACTIONS
The following adverse reactions associated with the use of phenylephrine hydrochloride were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably or to establish a causal relationship to drug exposure.
Cardiac disorders: Bradycardia, AV block, ventricular extrasystoles, myocardial ischemia
Gastrointestinal disorders: Nausea, vomiting
General disorders and administrative site conditions: Chest pain, extravasation
Immune system disorders: Sulfite sensitivity
Nervous system disorders: Headache, nervousness, paresthesia, tremor
Psychiatric disorders: Excitability
Respiratory: Pulmonary edema, rales
Skin and subcutaneous tissue disorders: Diaphoresis, pallor, piloerection, skin blanching, skin necrosis with extravasation
Vascular disorders: Hypertensive crisis
7 DRUG INTERACTIONS
7.1 Agonists
The pressor effect of phenylephrine hydrochloride is increased in patients receiving:
- Monoamine oxidase inhibitors (MAOI), such as selegiline.
- β-adrenergic blockers
- α-2 adrenergic agonists, such as clonidine
- Steroids
- Tricyclic antidepressants
- Norepinephrine transport inhibitors, such as atomoxetine
- Ergot alkaloids, such as methylergonovine maleate
- Centrally-acting sympatholytic agents, such as guanfacine or reserpine
- Atropine sulfate
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with intravenous phenylephrine. It is also not known whether phenylephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Phenylephrine hydrochloride should be given to a pregnant woman only if clearly needed.
8.2 Labor and Delivery
The most common maternal adverse reactions reported in studies of phenylephrine use during neuraxial anesthesia during cesarean delivery include nausea and vomiting, which are commonly associated with hypotension, bradycardia, reactive hypertension, and transient arrhythmias. Phenylephrine does not appear to cause a decrease in placental perfusion sufficient to alter either the neonate Apgar scores or blood-gas status.
8.5 Geriatric Use
Clinical studies of phenylephrine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Overdose of phenylephrine hydrochloride can cause a rapid rise in blood pressure. Symptoms of overdose include headache, vomiting, hypertension, reflex bradycardia, and cardiac arrhythmias including ventricular extrasystoles and ventricular tachycardia, and may cause a sensation of fullness in the head and tingling of the extremities. Consider using an α-adrenergic antagonist.
11 DESCRIPTION
Phenylephrine hydrochloride is a synthetic sympathomimetic agent in sterile form for parenteral injection. Chemically, phenylephrine hydrochloride is (-)-m-Hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride and has the following structural formula:..
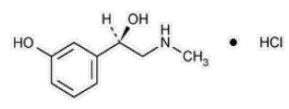
Phenylephrine hydrochloride is very soluble in water, freely soluble in ethanol, and insoluble in chloroform and ethyl ether. Phenylephrine hydrochloride is sensitive to light.
Phenylephrine hydrochloride injection, USP 10 mg/ml is a clear, colorless, aqueous solution that is essentially free of visible foreign matter. Each mL contains: Phenylephrine Hydrochloride 10 mg; Sodium Chloride 3.5 mg; Sodium Citrate Dihydrate 4 mg; Citric Acid Monohydrate 1 mg; and Sodium Metabisulfite 2 mg in water for injection. The pH may be adjusted in the range of 3.0 to 6.5 with Sodium Hydroxide and/or Hydrochloric Acid, if necessary.
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Phenylephrine is the active moiety. Metabolites are inactive at both the α-1and α-2 adrenergic receptors. Following parenteral administration of phenylephrine hydrochloride, increases in systolic blood pressure, diastolic blood pressure, mean arterial blood pressure, and total peripheral vascular resistance are observed. The onset of blood pressure increase following an intravenous bolus phenylephrine hydrochloride administration is rapid and the effect may persist for up to 20 minutes. As mean arterial pressure increases following parenteral doses, vagal activity also increases, resulting in reflex bradycardia.
Most vascular beds are constricted, including renal, splanchnic, and hepatic.
12.3 Pharmacokinetics
Following an intravenous infusion of phenylephrine hydrochloride, the effective half-life was approximately 5 minutes. The steady-state volume of distribution (340 L) exceeded the body volume by a factor of 5, suggesting a high distribution into certain organ compartments. The average total serum clearance (2095 mL/min) was close to one-third of the cardiac output.
A mass balance study showed that phenylephrine is extensively metabolized by the liver with only 12% of the dose excreted unchanged in the urine. Deamination by monoamino oxidase is the primary metabolic pathway resulting in the formation of the major metabolite (m-hydroxymandelic acid) which accounts for 57% of the total administered dose.
14 CLINICAL STUDIES
Increases in systolic and mean blood pressure following administration of phenylephrine were observed in 42 literature-based studies in the perioperative setting, including 26 studies where phenylephrine was used in low-risk (ASA 1 and 2) pregnant women undergoing neuraxial anesthesia during cesarean delivery, 3 studies in non-obstetric surgery under neuraxial anesthesia, and 13 studies in patients undergoing surgery under general anesthesia. Mean arterial blood pressure increases were also observed in two double-blind, active-controlled studies in patients with septic shock.
16 HOW SUPPLIED/STORAGE AND HANDLING
Phenylephrine hydrochloride injection, USP 10 mg/ml, is supplied as follows:
- NDC 69097-534-31: 1 mL single dose vials
- NDC 69097-534-97: Carton of 25 vials (25 x 1 mL single dose vials)
Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Protect from light. Keep covered in carton until time of use. For single use only. Discard unused portion.
17 PATIENT COUNSELING INFORMATION
Inform patients, families, or caregivers that the primary side effect of phenylephrine hydrochloride injection is hypertension and rarely, hypertensive crisis. Patients may experience bradycardia (slow heart rate), which in some cases may produce heart block or other cardiac arrhythmias, extra ventricular beats, myocardial ischemia in patients with underlying cardiac disease, and pulmonary edema (fluid in the lungs) or rales. Common, less serious symptoms include the following:
- chest pain
- skin or tissue damage if the drug leaks out of the venous catheter into the surrounding tissue
- headache, nervousness, tremor, numbness/tingling (paresthesias) in hands or feet
- nausea, vomiting
- excitability, dizziness, sweating, flushing
Cipla Ltd.
Verna Goa, India
Manufactured for:
Cipla USA, Inc.
9100 S. Dadeland Blvd., Suite 1500
Miami, Florida 33156
Issued: 03/2017
| PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Cipla USA Inc. (078719707) |
| Registrant - Cipla USA Inc. (078719707) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cipla Ltd.- Goa | 650072015 | MANUFACTURE(69097-534) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Malladi Drugs and Pharmaceuticals Limited | 650474468 | ANALYSIS(69097-534) , API MANUFACTURE(69097-534) , MANUFACTURE(69097-534) , PACK(69097-534) | |
