DEXPANTHENOL- dexpanthenol injection, solution
American Regent, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
DEXPANTHENOL INJECTION
DESCRIPTION
Dexpanthenol is a derivative of pantothenic acid, a B complex vitamin. Dexpanthenol Injection is a sterile, nonpyrogenic, aqueous solution indicated for use as a gastrointestinal stimulant. The chemical name is D-(+)-2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethybutylamide. The structural formula is:
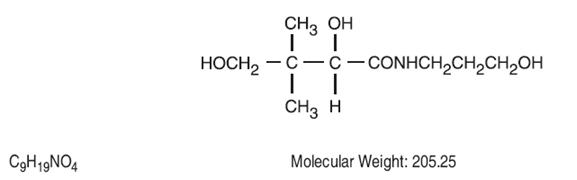
Each mL contains Dexpanthenol 250 mg in Water for Injection q.s. pH (range 4 - 7) may be adjusted with Sodium Citrate and/or Citric Acid.
CLINICAL PHARMACOLOGY
Pantothenic acid is a precursor of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions involving transfer of acetyl groups. The final step in the synthesis of acetylcholine consists of the choline acetylase transfer of acetyl group from acetylcoenzyme A to choline. Acetylcholine is the neurohumoral transmitter in the parasympathetic system and as such maintains the normal functions of the intestine. Decrease in acetylcholine content would result in decreased peristalsis and in extreme cases adynamic ileus. The pharmacological mode of action of the drug is unknown. Pharmacokinetics data in humans is unavailable.
INDICATIONS AND USAGE
Prophylactic use immediately after major abdominal surgery to minimize the possibility of paralytic ileus. Intestinal atony causing abdominal distention; postoperative or postpartum retention of flatus, or postoperative delay in resumption of intestinal motility; paralytic ileus.
WARNINGS
There have been rare instances of allergic reactions of unknown cause during the concomitant use of Dexpanthenol Injection with drugs such as antibiotics, narcotics and barbiturates.
Administration of Dexpanthenol Injection directly into the vein is not advised (See Dosage and Administration).
Dexpanthenol Injection should not be administered within one hour of succinylcholine.
PRECAUTIONS
General
If any signs of a hypersensitivity reaction appear, Dexpanthenol Injection should be discontinued. If ileus is a secondary consequence of mechanical obstruction, primary attention should be directed to the obstruction.
The management of adynamic ileus includes the correction of any fluid and electrolyte imbalance (especially hypokalemia), anemia and hypoproteinemia, treatment of infection, avoidance where possible of drugs which are known to decrease gastrointestinal motility and decompression of the gastrointestinal tract when considerably distended by nasogastric suction or use of a long intestinal tube.
Drug Interactions
The effects of succinylcholine appeared to have been prolonged in a woman administered dexpanthenol.
Carcinogenicity, Mutagenicity, and Impairment of Fertility - There have been no studies in animals to evaluate the carcinogenic, mutagenic, or impairment of fertility potential of dexpanthenol.
Pregnancy
Teratogenic Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with Dexpanthenol Injection. It is also not known whether Dexpanthenol Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Dexpanthenol Injection should be given to a pregnant
woman only if clearly needed.
ADVERSE REACTIONS
There have been a few reports of allergic reactions and single reports of several other adverse events in association with the administration of dexpanthenol. A causal relationship is uncertain. One patient experienced itching, tingling, difficulty in breathing. Another patient had red patches of skin. Two patients had generalized dermatitis and one patient urticaria.
One patient experienced temporary respiratory difficulty following administration of dexpanthenol injection 5 minutes after succinylcholine was discontinued.
One patient experienced a noticeable but slight drop in blood pressure after administration of dexpanthenol while in the recovery room.
One patient experienced intestinal colic one-half hour after the drug was administered.
Two patients vomited following administration and two patients had diarrhea 10 days post-surgery and after Dexpanthenol Injection.
One elderly patient became agitated after administration of the drug.
DOSAGE AND ADMINISTRATION
Prevention of post-operative adynamic ileus: 250 mg (1 mL) or 500 mg (2 mL) intramuscularly. Repeat in 2 hours and then every 6 hours until all danger of adynamic ileus has passed.
Treatment of adynamic ileus: 500 mg (2 mL) intramuscularly. Repeat in 2 hours and then every 6 hours as needed.
Intravenous administration: Dexpanthenol Injection 2 mL (500 mg) may be mixed with bulk I.V. solutions such as glucose or Lactated Ringer’s and slowly infused intravenously.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
Dexpanthenol Injection 250 mg/mL
NDC 0517-0131-25 2 mL Single Dose Vial Packed in boxes of 25
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
IN0131
Rev. 1/09
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 2 mL Carton
DEXPANTHENOL
INJECTION
250 mg/mL
NDC 0517-0131-25
25 X 2 mL SINGLE DOSE VIALS
FOR INTRAMUSCULAR USE.
FOR INTRAVENOUS USE AFTER DILUTION.
Rx Only
Each mL contains: Dexpanthenol 250 mg in Water for Injection q.s. pH may be adjusted with Sodium Citrate and/or Citric Acid. Sterile, nonpyrogenic. WARNING: DISCARD UNUSED PORTION. Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 7/06

| DEXPANTHENOL
dexpanthenol injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Regent, Inc. (622781813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Luitpold Pharmaceuticals, Inc. | 002033710 | ANALYSIS(0517-0131) , MANUFACTURE(0517-0131) , STERILIZE(0517-0131) | |