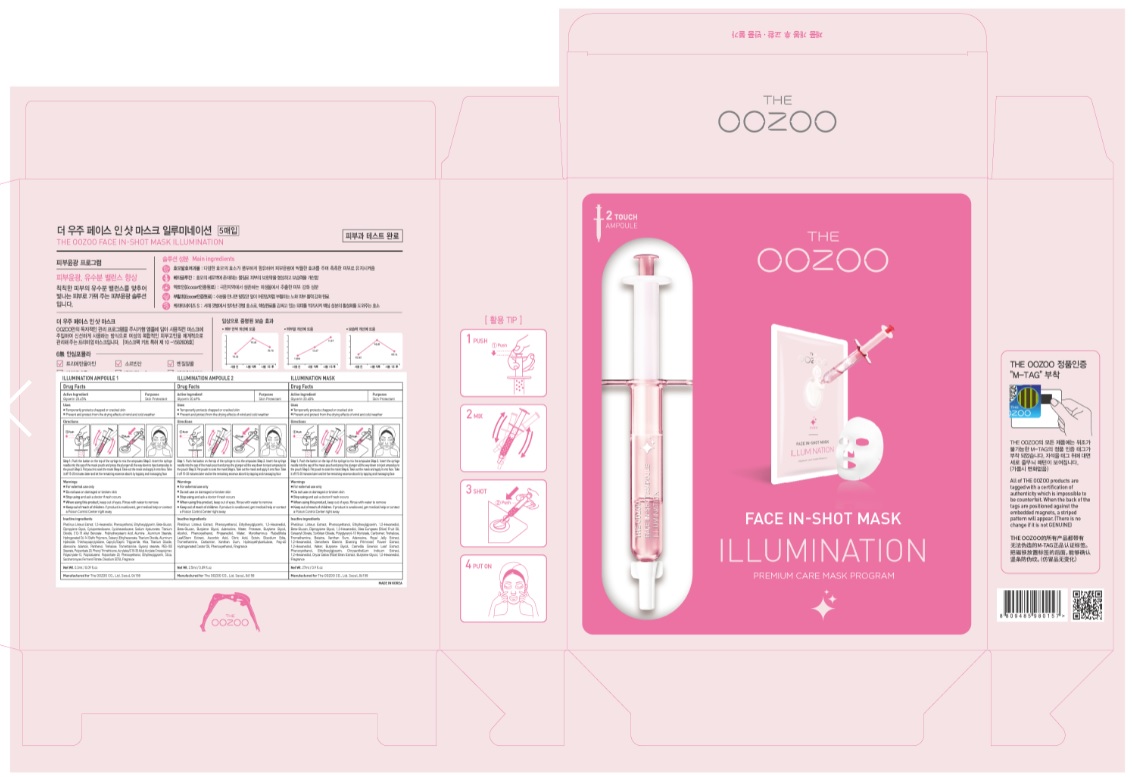
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 1- glycerin liquid
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 2- glycerin liquid
OOZOO FACE IN-SHOT MASK ILLUMINATION MASK- glycerin patch
OUTIN FUTURES CORP.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 1
Glycerin 20.45%
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 2
Glycerin 20.69%
OOZOO FACE IN-SHOT MASK ILLUMINATION MASK
Glycerin 20.60%
Temporarily protects chapped or cracked skin
Prevent and protect from the drying effects of wind and cold weather
Step 1. Push the button on the top of the syringe to mix the ampoules
Step 2. Insert the syringe needle into the cap of the mask pouch and press the plunger all the way down to inject ampoules to the pouch
Step 3. Pat pouch to soak the mask
Step 4. Take out the mask and apply it onto face. Take it off 15-20 minutes later and let the remaining essence absorb by tapping and massaging face
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop using and ask a doctor if rash occurs.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 1
Phellinus Linteus Extract, 1,2-Hexanediol, Phenoxyethanol, Ethylhexylglycerin, Beta-Glucan, Dipropylene Glycol, Cyclopentasiloxane, Cyclohexasiloxane, Sodium Hyaluronate, Titanium Dioxide, C12-15 Alkyl Benzoate , Polyhydroxystearic Acid , Alumina , Aluminum Stearate, Hydrogenated C6-14 Olefin Polymers, Cetearyl Ethylhexanoate, Titanium Dioxide, Aluminum Hydroxide, Triethoxycaprylylsilane, Caprylic/Capric Triglyceride, Mica, Titanium Dioxide, Adenosine, Allantoin, Panthenol, Trehalose, Tromethamine, Glyceryl stearate, PEG-100 Stearate, Polysorbate 20, Phenyl Trimethicone, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyacrylate-13, Polyisobutene, Polysorbate 20, Phenoxyethanol, Ethylhexylglycerin, Silica, Saccharomyces Ferment Filtrate, Disodium EDTA, Fragrance
OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 2
Phellinus Linteus Extract, Phenoxyethanol, Ethylhexylglycerin, 1,2-Hexanediol, Beta-Glucan, Butylene Glycol, Adenosine, Water, Protease, Butylene Glycol, Alcohol, Phenoxyethanol, Propanediol, Water, Myrothamnus Flabellifolia Leaf/Stem Extract, Ascorbic Acid, Citric Acid, Ectoin, Disodium Edta, Tromethamine, Carbomer, Xanthan Gum, Hydroxyethylcellulose, Peg-60 Hydrogenated Castor Oil, Phenoxyethanol, Fragrance
OOZOO FACE IN-SHOT MASK ILLUMINATION MASK
Phellinus Linteus Extract, Phenoxyethanol, Ethylhexylglycerin, 1,2-Hexanediol, Beta-Glucan, Dipropylene Glycol, 1,2-Hexanediol, Olea Europaea (Olive) Fruit Oil, Cetearyl Olivate, Sorbitan Olivate, Polyglyceryl-10 Myristate, Carbomer, Trehalose, Tromethamine, Betaine, Xanthan Gum, Adenosine, Royal Jelly Extract, 1,2-Hexanediol, Oenothera Biennis (Evening Primrose) Flower Extract, 1,2-Hexanediol, Water, Butylene Glycol, Camellia Sinensis Leaf Extract, Phenoxyethanol, Ethylhexylglycerin, Chrysanthellum Indicum Extract, 1,2-Hexanediol, Oryza Sativa (Rice) Bran Extract, Butylene Glycol, 1,2-Hexanediol, Fragrance
| OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 1
glycerin liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OOZOO FACE IN-SHOT MASK ILLUMINATION AMPOULE 2
glycerin liquid |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| OOZOO FACE IN-SHOT MASK ILLUMINATION MASK
glycerin patch |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - OUTIN FUTURES CORP. (688864073) |
| Registrant - OUTIN FUTURES CORP. (688864073) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OUTIN FUTURES CORP. | 689851514 | manufacture(69924-031, 69924-032, 69924-033) | |