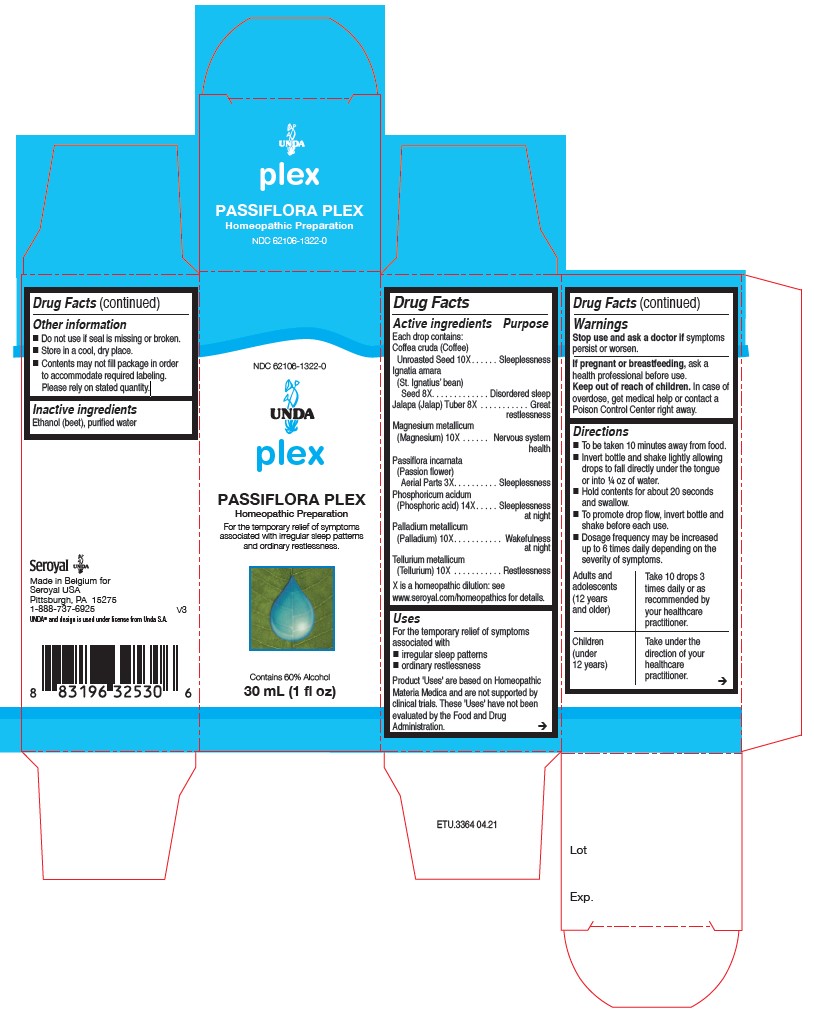
Label: PASSIFLORA PLEX- passiflora incarnata, ignatia amara, jalapa, coffea cruda, magnesium metalicum, palladium metallicum, tellurium metallicum, phosphoricum acidum liquid
- NDC Code(s): 62106-1322-0
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Coffea cruda (Coffee) Seed 10X
Ignatia amara (St. Ignatius’ bean) Seed 8X
Jalapa (Jalap) Tuber 8X
Magnesium metallicum (Magnesium) 10X
Passiflora incarnata (Passion flower) Aerial Parts 3X
Phosphoricum acidum (Phosphoric acid) 14X
Palladium metallicum (Palladium) 10X
Tellurium metallicum (Tellurium) 10X - PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions
To be taken ten minutes away from food.
Invert bottle and shake lightly allowing drops to fall directly under the tongue or into ¼ oz of water.
Hold contents for about 20 seconds and swallow.
To promote drop flow, invert bottle and shake before each use.
Dosage frequency may be increased up to six times daily depending on the severity of symptoms.Adults and adolescents (12 years and older)
Take 10 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with
irregular sleep patterns
ordinary restlessnessDirections
To be taken ten minutes away from food.
Invert bottle and shake lightly allowing drops to fall directly under the tongue or into ¼ oz of water.
Hold contents for about 20 seconds and swallow.
To promote drop flow, invert bottle and shake before each use.
Dosage frequency may be increased up to six times daily depending on the severity of symptoms.Adults and adolescents (12 years and older)
Take 10 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner.
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PASSIFLORA PLEX
passiflora incarnata, ignatia amara, jalapa, coffea cruda, magnesium metalicum, palladium metallicum, tellurium metallicum, phosphoricum acidum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1322 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 3 [hp_X] in 30 mL IPOMOEA PURGA ROOT (UNII: 4UDO46YBK2) (IPOMOEA PURGA ROOT - UNII:4UDO46YBK2) IPOMOEA PURGA ROOT 8 [hp_X] in 30 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 10 [hp_X] in 30 mL MAGNESIUM (UNII: I38ZP9992A) (MAGNESIUM - UNII:I38ZP9992A) MAGNESIUM 10 [hp_X] in 30 mL PALLADIUM (UNII: 5TWQ1V240M) (PALLADIUM - UNII:5TWQ1V240M) PALLADIUM 10 [hp_X] in 30 mL TELLURIUM (UNII: NQA0O090ZJ) (TELLURIUM - UNII:NQA0O090ZJ) TELLURIUM 10 [hp_X] in 30 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 14 [hp_X] in 30 mL STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 8 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1322-0 1 in 1 BOX 02/09/2015 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/09/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-1322)