FLUOXETINE - fluoxetine hydrochloride capsule
Lake Erie Medical DBA Quality Care Products LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use fluoxetine safely and effectively. See full prescribing information for fluoxetine capsules.
Fluoxetine Capsules, USP for Oral Use Initial U.S. Approval: 1987 WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS See full prescribing information for complete boxed warning. Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants for Major Depressive Disorder (MDD) and other psychiatric disorders (5.1). When using fluoxetine and olanzapine in combination, also refer to Boxed Warning section of the package insert for Symbyax. RECENT MAJOR CHANGES
Indications and Usage, Fluoxetine Capsules and Olanzapine in Combination: Revised: 1/2017 |
FULL PRESCRIBING INFORMATION: CONTENTS*WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS11 DESCRIPTION
|
FULL PRESCRIBING INFORMATION
WARNING: SUICIDALITY AND ANTIDEPRESSANT DRUGS
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of fluoxetine or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Fluoxetine is approved for use in pediatric patients with MDD and Obsessive Compulsive Disorder (OCD) [see Warnings and Precautions (5.1) and Use in Specific Populations ( 8.4)].
When using fluoxetine and olanzapine in combination, also refer to Boxed Warning section of the package insert for Symbyax.
11 DESCRIPTION
Fluoxetine capsules, USP are a selective serotonin reuptake inhibitor for oral administration. It is also marketed for the treatment of premenstrual dysphoric disorder (Sarafem®, fluoxetine hydrochloride). It is designated (±)-N-methyl-3-phenyl-3-[(α,α,α-trifluoro-p-tolyl)oxy]propylamine hydrochloride and has the molecular formula of C17H18F3NO•HCl. Its molecular weight is 345.79. The structural formula is:
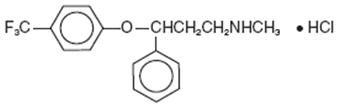
Fluoxetine hydrochloride is a white to off-white crystalline solid with a solubility of 14 mg/mL in water.
Each capsule contains fluoxetine hydrochloride equivalent to 10 mg (32.3 μmol), 20 mg (64.7 μmol), or 40 mg (129.3 μmol) of fluoxetine. The capsules also contain the following inactive ingredients: pregelatinized starch, colloidal silicon dioxide, FD&C Blue #1, yellow iron oxide, titanium dioxide, sodium lauryl sulphate, and gelatin. In addition 40 mg also contains FD&C Yellow #6. The capsules are printed with edible ink containing black iron oxide and shellac.
| FLUOXETINE
fluoxetine hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Lake Erie Medical DBA Quality Care Products LLC (831276758) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lake Erie Medical DBA Quality Care Products LLC | 831276758 | repack(49999-362) | |
