FOR PRESCRIPTION COMPOUNDING ONLY
DESCRIPTION
Each Nazirex Compounding Kit provides 3 grams of Levocetirizine Dihydrochloride USP, 3 grams of Loratadine USP, and 54 grams of Base. The resulting mixture is intended for topical use.
TO THE PHARMACIST:
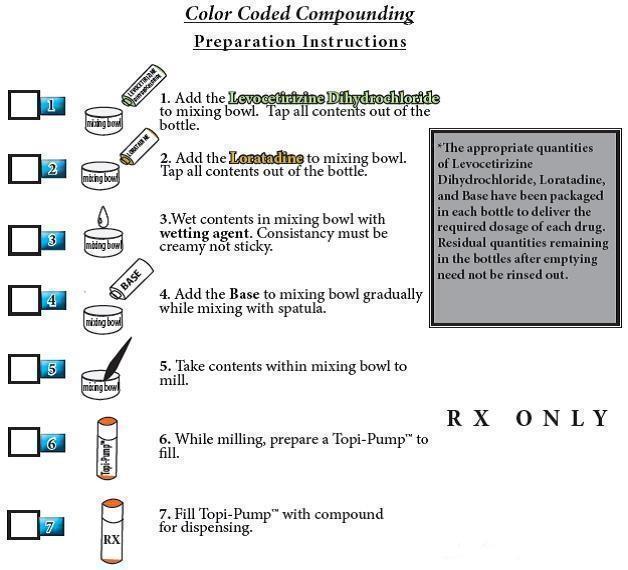
Kit Components: 1 color coded light green bottle of 3g of Levocetirizine Dihydrochloride, 1 color coded sand bottle of 3g of Loratadine, and 1 bottle of 54g Base.
Equipment needed: mill, spatula, mixing bowl, and Topi-Pump
TM.
| SIZE | 60 grams |
| NDC # | 15455-9003-9 |
| Levocetirizine Dihydrochloride, USP | 3 grams |
| Loratadine, USP | 3 grams |
| Base | 54 grams |
Prior to compounding, store Nazirex Compounding Kit at room temperature between 20 - 25 degrees C (68 - 77 degrees F). Protect from light.
Nazirex Compounding Kit components have a two-year expiration date.
For external use only. Avoid contact with eyes. Keep container tightly closed. Keep out of reach of children. Protect from light. Dispose of product after 30 days of being compounded.
* Certificate of analysis on file.
The FDA has not approved Nazirex to cure, treat, or mitigate disease.
Nazirex is intended for preparation in accordance with state and federal regulation governing compounding and is available to patients by prescription only.
Rx ONLY
Alvix Laboratories, LLC
Ocean Springs, MS 39564
1(888)526-5449
www.Alvix.com

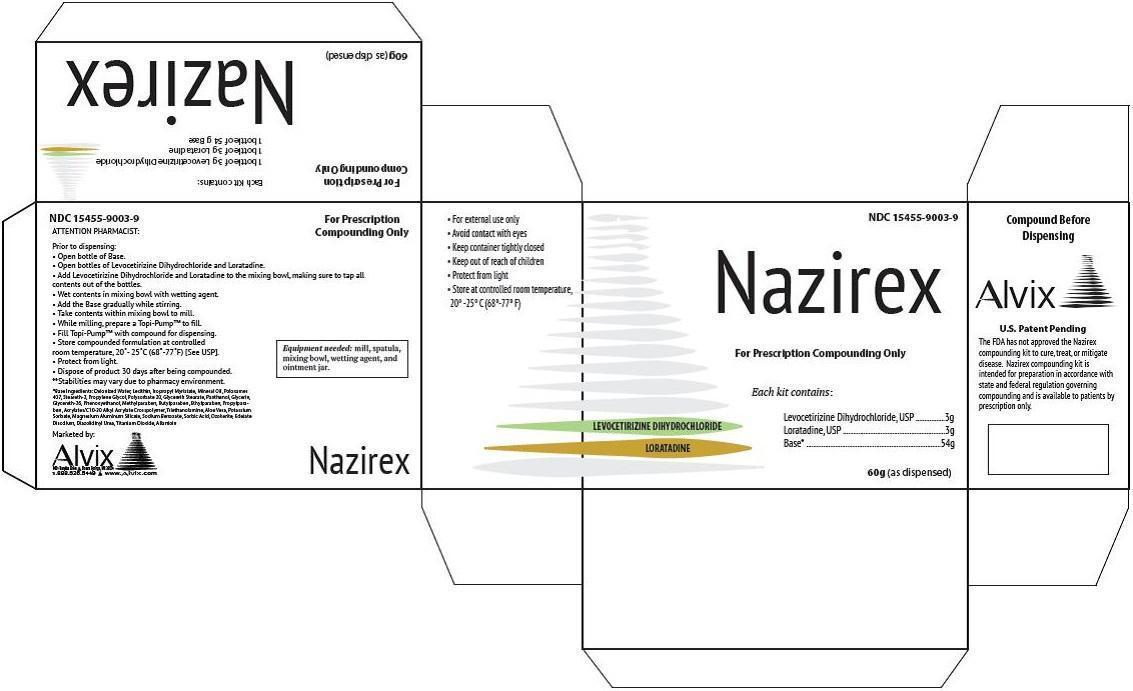
PRINCIPAL DISPLAY PANEL
NDC 15455-9003-9
RX ONLY
Nazirex Compounding Kit
Levocetirizine Dihydrochloride, USP ..... 3 grams
Loratadine, USP .................................. 3 grams
Cream Base ....................................... 54 grams
FOR PRESCRIPTION COMPOUNDING ONLY
60 grams as dispensed