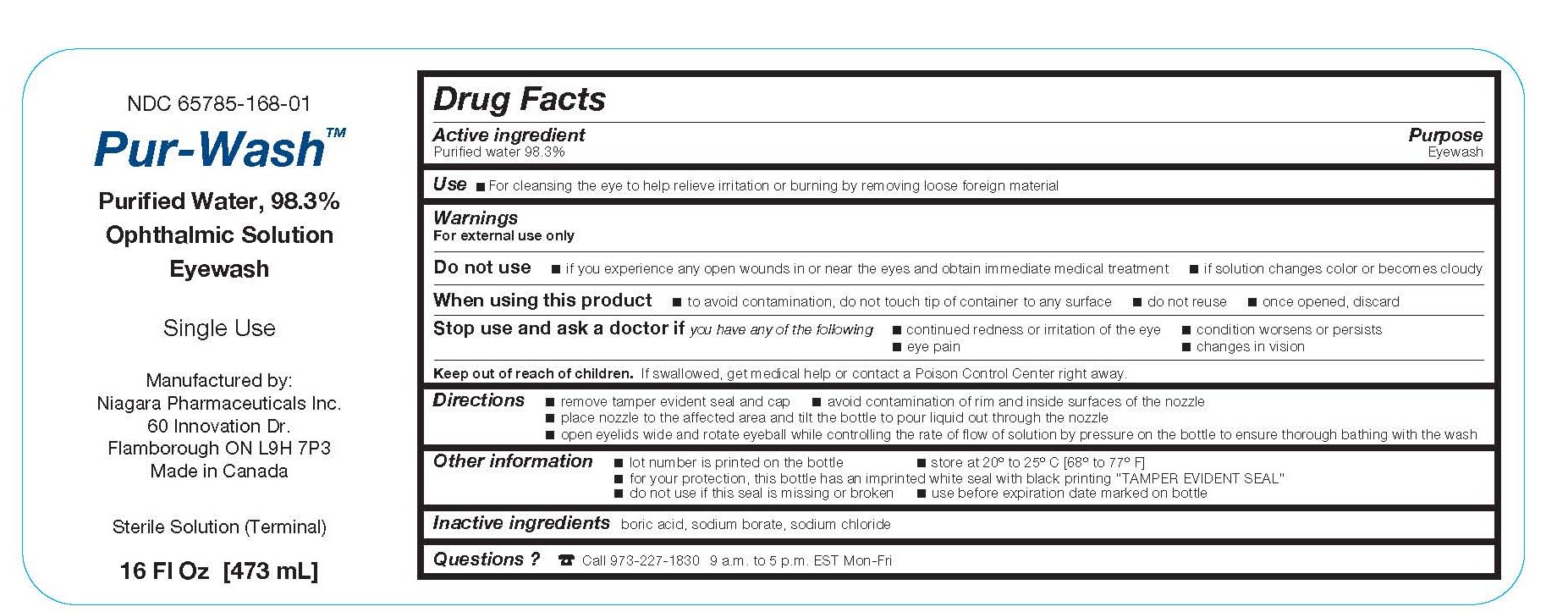
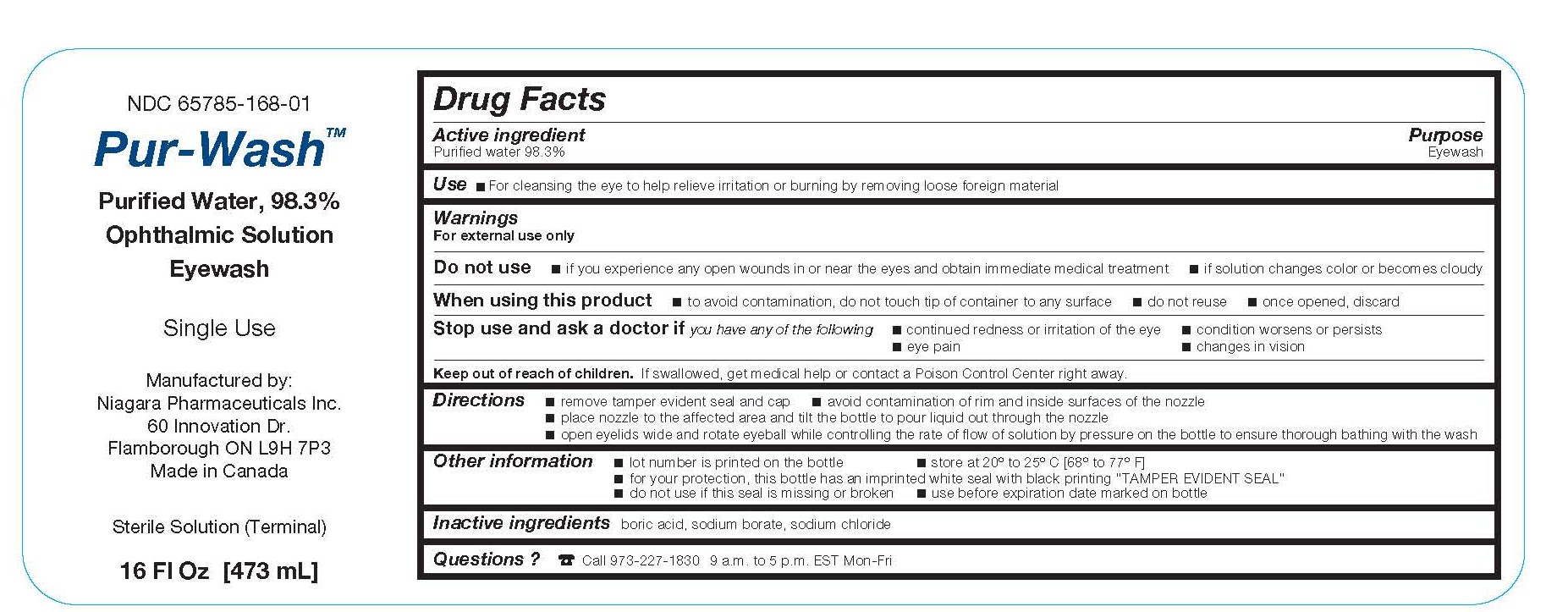
Label: PUR-WASH- eyewash solution
- NDC Code(s): 65785-168-01, 65785-168-02
- Packager: Niagara Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Warnings
- Use
- Stop use and ask a doctor if you have any of the following
- Keep out of reach of children
-
Directions
- remove tamper evident seal and cap
- avoid contamination of rim and inside surfaces of the nozzle
- place nozzle to the affected area and tilt the bottle to pour liquid out through the nozzle
- open eyelids wide and rotate eyeball while controlling the rate of flow of solution by pressure on the bottle to ensure thorough bathing with the wash
- Other information
- Inactive ingredients
- Questions?
-
Dosage and Administration
- remove tamper evident seal and cap
- avoid contamination of rim and inside surfaces of the nozzle
- place nozzle to the affected area and tilt the bottle to pour liquid out through the nozzle
- open eyelids wide and rotate eyeball while controlling the rate of flow of solution by pressure on the bottle to ensure thorough bathing with the wash
- Warnings
- Package Label.Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PUR-WASH
eyewash solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65785-168 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 465 mL in 473 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65785-168-01 473 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 2 NDC:65785-168-02 946 mL in 1 BOTTLE, UNIT-DOSE; Type 0: Not a Combination Product 09/01/2015 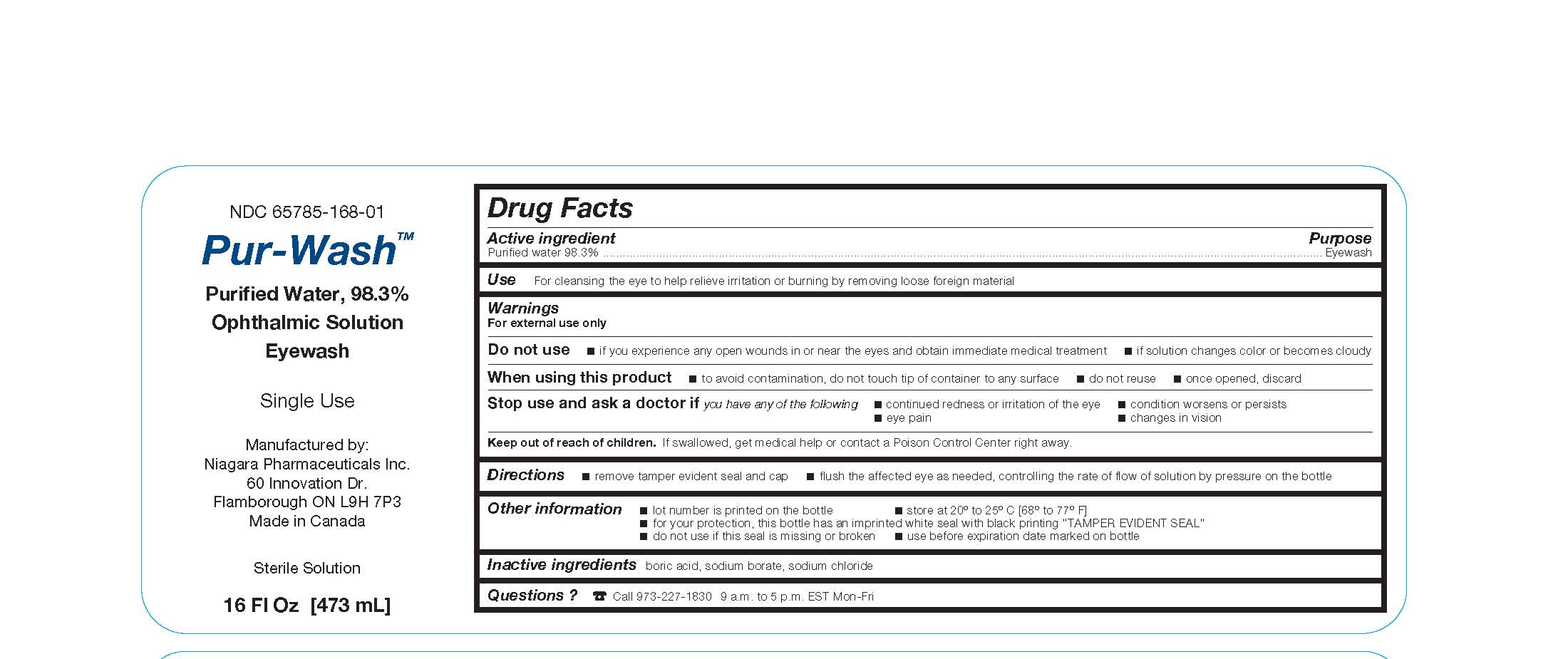
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 01/30/2015 Labeler - Niagara Pharmaceuticals, Inc. (205477792) Registrant - Niagara Pharmaceuticals, Inc. (205477792) Establishment Name Address ID/FEI Business Operations Niagara Pharmaceuticals, Inc. 205477792 manufacture(65785-168)