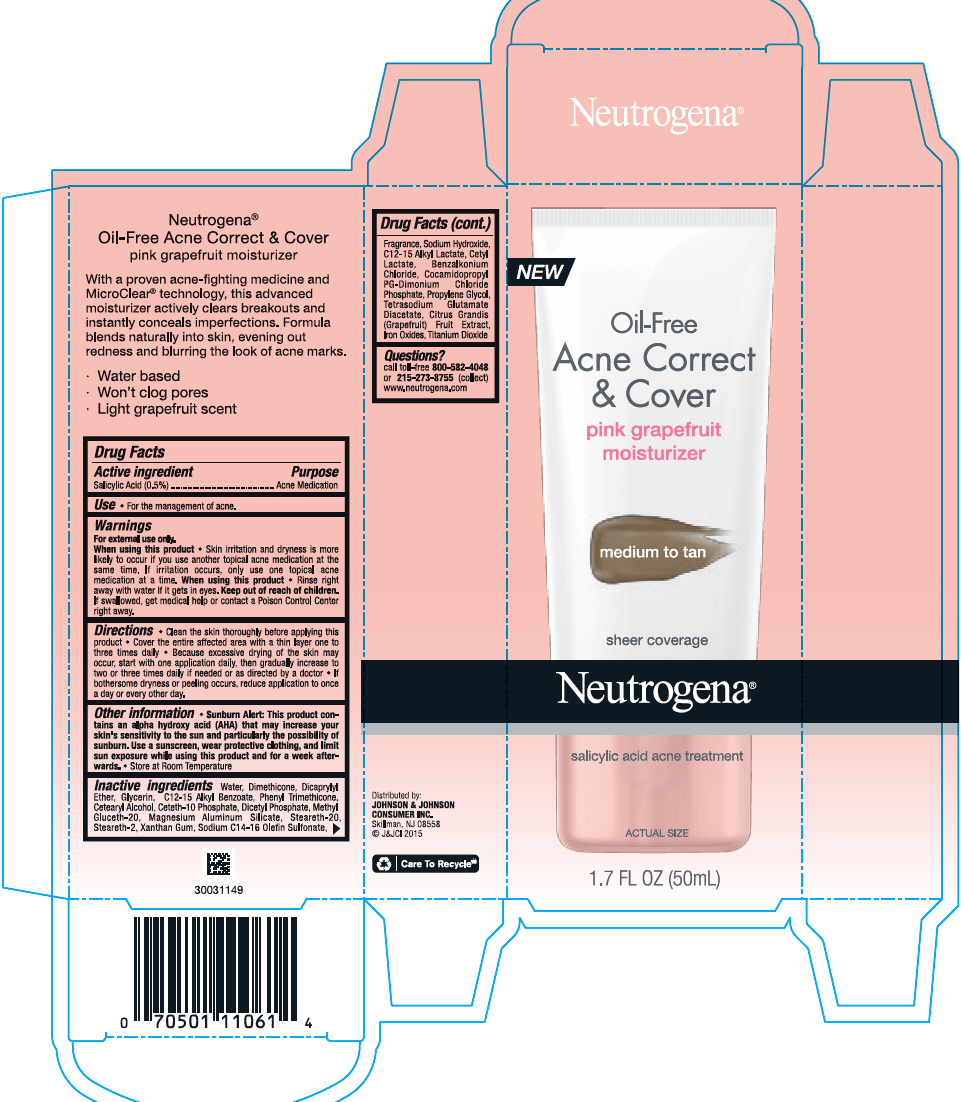
NEUTROGENA OIL FREE ACNE CORRECT AND COVER PINK GRAPEFRUIT MOISTURIZER MEDIUM TO TAN- salicylic acid lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neutrogena Oil Free Acne Moisturizer Pink Grapefruit Correct & Cover
(medium to tan)
Warnings
For external use only.
Directions
- Clean the skin thoroughly before applying this product
- Cover the entire affected area with a thin layer one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Other Information
- Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards.
- Store at Room Temperature
Inactive Ingredients
Water, Dimethicone, Dicaprylyl Ether, Glycerin, C12-15 Alkyl Benzoate, Phenyl Trimethicone, Cetearyl Alcohol, Ceteth-10 Phosphate, Dicetyl Phosphate, Methyl Gluceth-20, Magnesium Aluminum Silicate, Steareth-20, Steareth-2, Xanthan Gum, Sodium C14-16 Olefin Sulfonate, Fragrance, Sodium Hydroxide, C12-15 Alkyl Lactate, Cetyl Lactate, Benzalkonium Chloride, Cocamidopropyl PG-Dimonium Chloride Phosphate, Propylene Glycol, Tetrasodium Glutamate Diacetate, Citrus Grandis (Grapefruit) Fruit Extract, Iron Oxides, Titanium Dioxide
| NEUTROGENA OIL FREE ACNE CORRECT AND COVER PINK GRAPEFRUIT MOISTURIZER
MEDIUM TO TAN
salicylic acid lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |