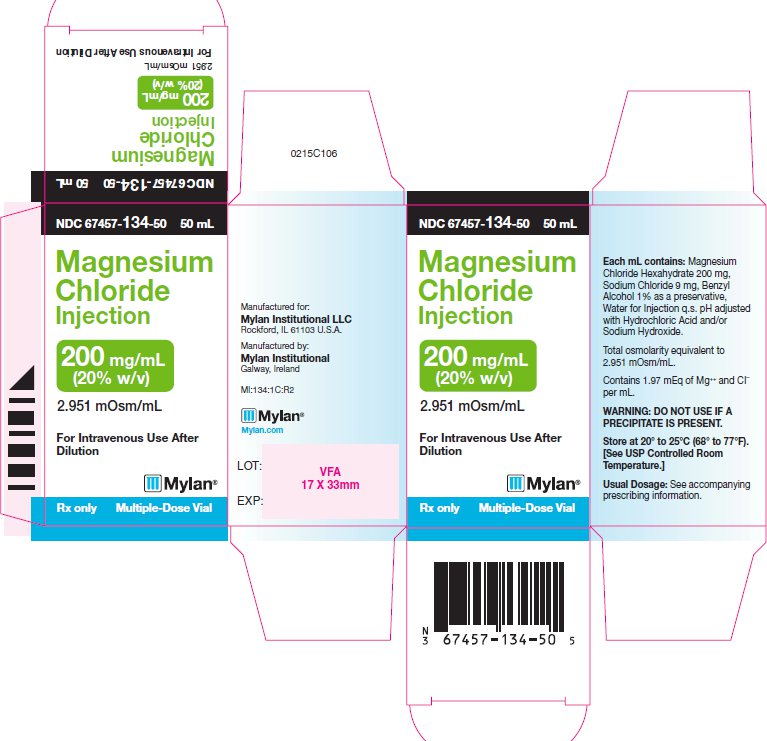
MAGNESIUM CHLORIDE- magnesium chloride injection
Mylan Teoranta
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
| MAGNESIUM CHLORIDE
magnesium chloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Teoranta (896321325) |
Revised: 3/2013
Document Id: ef734081-c73d-4d17-bd29-7a02b9256c7b
Set id: 0cb8e560-636f-4127-b802-cdfd8f23147f
Version: 2
Effective Time: 20130314
Mylan Teoranta