Label: ACETIC ACID solution
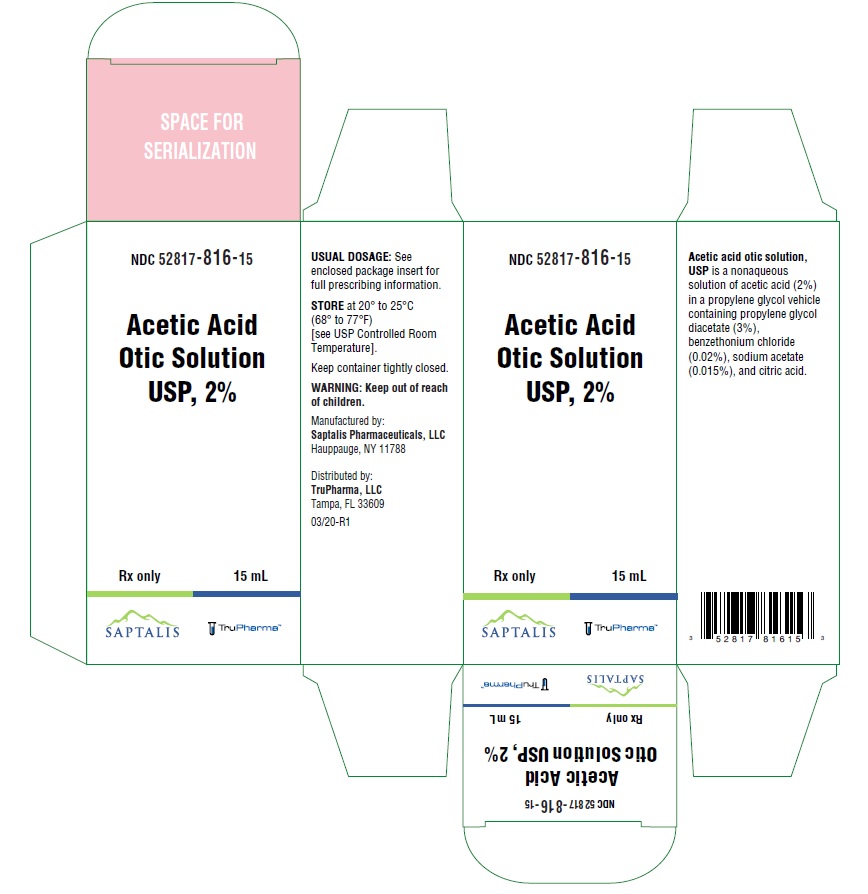
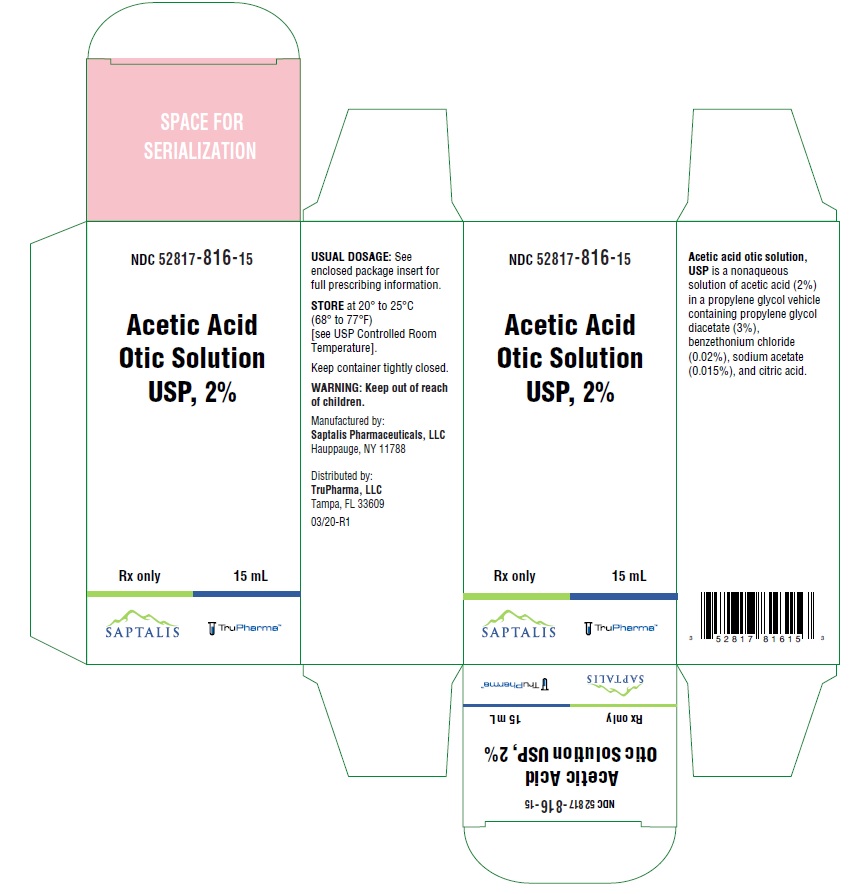
- NDC Code(s): 52817-816-15
- Packager: TruPharma, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 24, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Rx only
-
DESCRIPTION
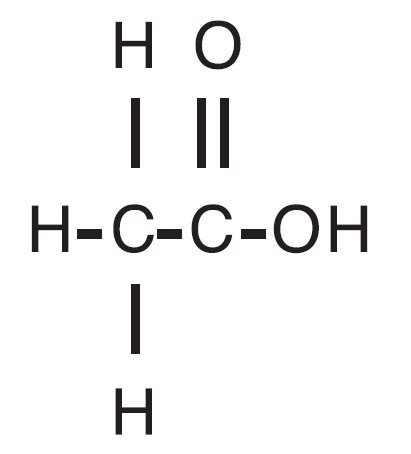
Acetic acid otic solution, USP is a solution of acetic acid (2%), in a propylene glycol vehicle containing propylene glycol diacetate (3%), benzethonium chloride (0.02%), sodium acetate (0.015%), and citric acid. The molecular formula for acetic acid is CH3COOH, with a molecular weight of 60.05. The structural formula is:
Acetic acid otic solution, USP is available as a nonaqueous otic solution buffered at pH 3 for use in the external ear canal.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PEDIATRIC USE
- ADVERSE REACTIONS
-
DOSAGE AND ADMINISTRATION
Carefully remove all cerumen and debris to allow acetic acid otic solution to contact infected surfaces directly. To promote continuous contact, insert a wick of cotton saturated with acetic acid otic solution into the ear canal; the wick may also be saturated after insertion. Instruct the patient to keep the wick in for at least 24 hours and to keep it moist by adding 3 drops to 5 drops of acetic acid otic solution every 4 hours to 6 hours. The wick may be removed after 24 hours but the patient should continue to instill 5 drops of acetic acid otic solution 3 times or 4 times daily thereafter, for as long as indicated. In pediatric patients, 3 drops to 4 drops may be sufficient due to the smaller capacity of the ear canal.
- HOW SUPPLIED
- STORAGE
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETIC ACID
acetic acid solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52817-816 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETIC ACID (UNII: Q40Q9N063P) (ACETIC ACID - UNII:Q40Q9N063P) ACETIC ACID 20.65 mg in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZETHONIUM CHLORIDE (UNII: PH41D05744) PROPYLENE GLYCOL DIACETATE (UNII: 5Z492UNF9O) SODIUM ACETATE (UNII: 4550K0SC9B) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52817-816-15 1 in 1 CARTON 06/05/2020 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040607 06/05/2020 Labeler - TruPharma, LLC (078533947) Registrant - Saptalis Pharmaceuticals, LLC (080145868) Establishment Name Address ID/FEI Business Operations Saptalis Pharmaceuticals, LLC 081154447 MANUFACTURE(52817-816)