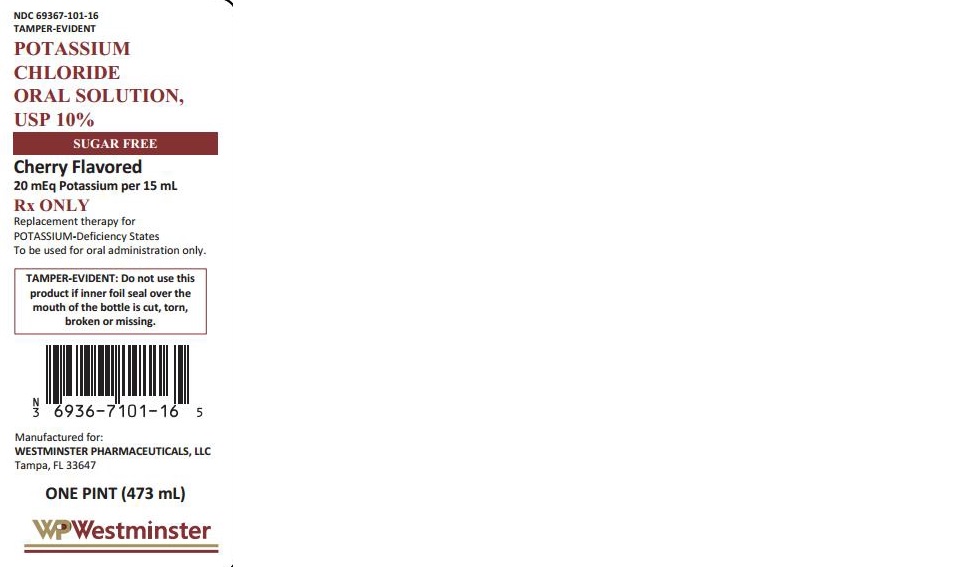
POTASSIUM CHLORIDE- potassium chloride solution
Westminster Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Potassium Chloride Oral Solution, USP 10 %
INSTRUCTIONS FOR USE SECTION
To minimize gastrointestinal irritation, patients must follow direction regarding dilution. Each tablespoon (15mL) should be diluted with three (3) fluid ounce or more of water or other liquid.
DOSAGE FORM AND STRENGTH
Adult Dosage: one (1) tablespoon (15mL) twice daily (after morning or evening meals) supplies 40mEq of potassium.
| POTASSIUM CHLORIDE
potassium chloride solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Westminster Pharmaceuticals, LLC (079516651) |
Revised: 10/2015
Document Id: 2124cba8-c932-4ffe-e054-00144ff8d46c
Set id: 0b35cd59-0caa-4ba5-e054-00144ff8d46c
Version: 5
Effective Time: 20151002
Westminster Pharmaceuticals, LLC