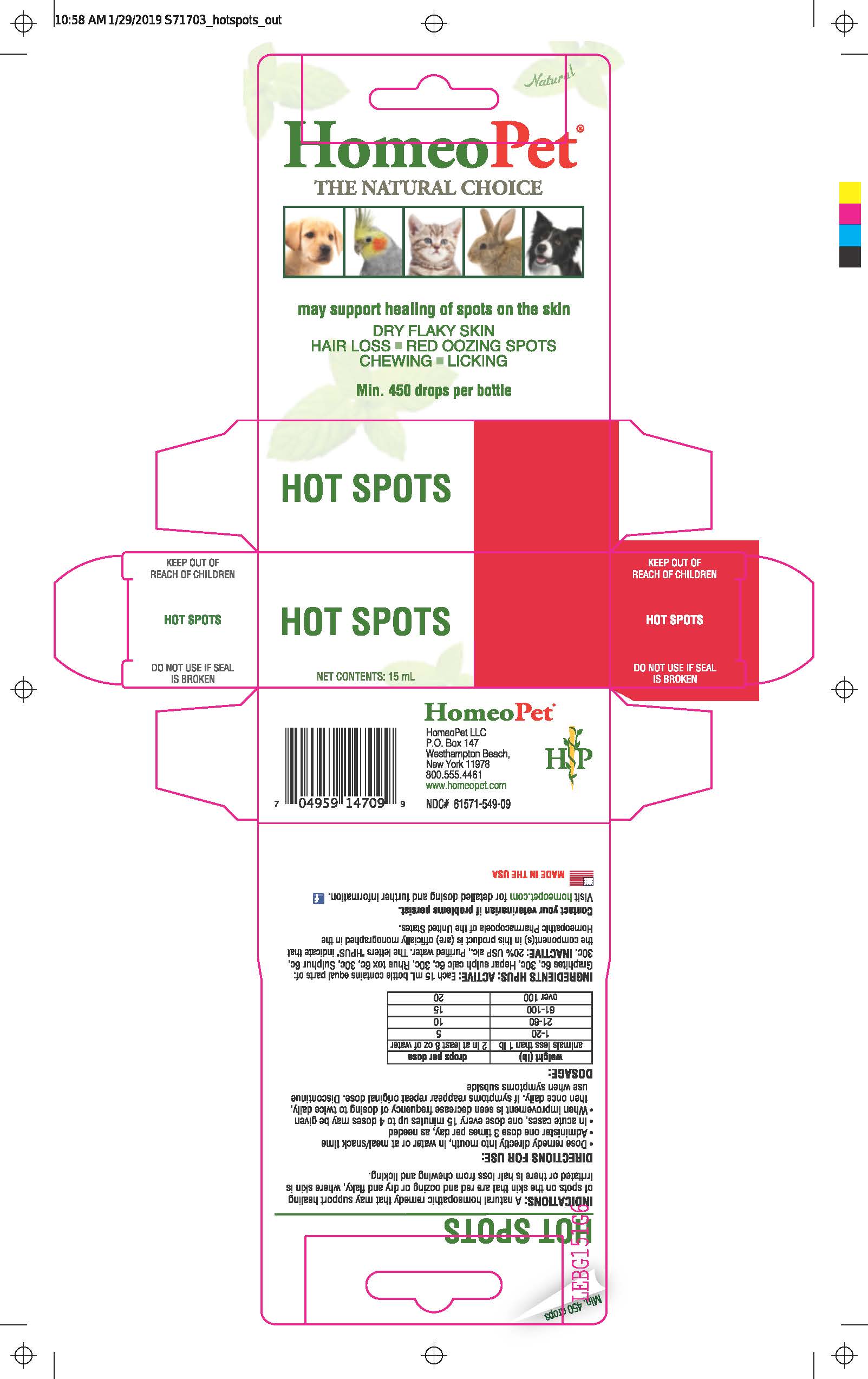
Label: HOT SPOTS- rhus toxicodendron, sulphur, hepar sulphuris calcareum, graphites liquid
- NDC Code(s): 61571-549-09
- Packager: HomeoPet, LLC
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 28, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Hot Spots
-
Directions for Use and Dosage
- Dose remedy directly into mouth, in water or at meal/snack time
- Administer one dose 3 times per day, as needed
- In acute cases, one dose every 15 minutes up to 4 doses may be given
- When improvement is seen decrease frequency of dosing to twice daily, then once daily. If symptoms reappear repeat original dose. Discontinue use when symptoms subside
weight (lb)
drops per dose
animals less than 1 lb
2 in at least 8 oz of water
1-20
5
21-60
10
61-100
15
over 100
20
- Contact Veterinarian
- Ingredients HPUS: Active
- Inactive Ingredients
- Indications
- Hot Spot Box
-
INGREDIENTS AND APPEARANCE
HOT SPOTS
rhus toxicodendron, sulphur, hepar sulphuris calcareum, graphites liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:61571-549 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 6 [hp_C] in 15 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 6 [hp_C] in 15 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_C] in 15 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_C] in 15 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61571-549-09 15 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/15/1995 Labeler - HomeoPet, LLC (121272657) Registrant - HomeoPet, LLC (121272657) Establishment Name Address ID/FEI Business Operations Speciality Pharma Manufacturing LLC 013957125 api manufacture, manufacture