OXY SKIN CLEARING SOOTHING CLEANSER DAILY DEFENSE- salicylic acid liquid
The Mentholatum Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
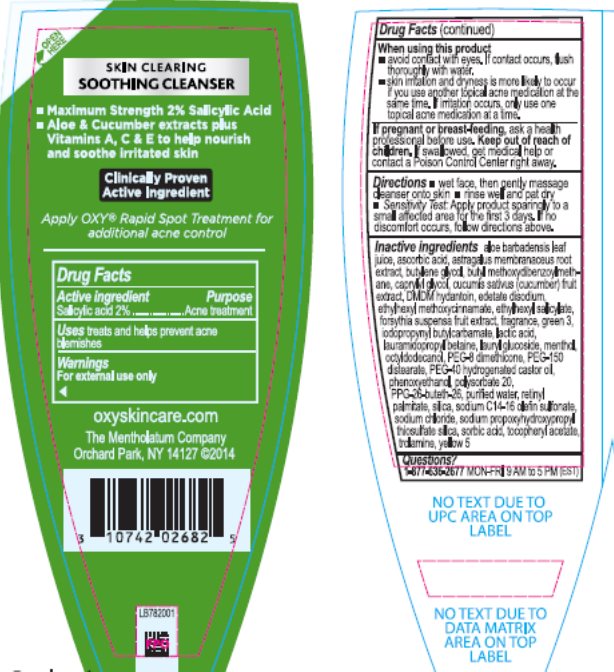
Drug Facts
Warnings
For external use only
Directions
- •
- wet face, then gently massage cleanser onto skin
- •
- rinse well and pat dry
- •
- Sensitivity Test: Apply product sparingly to a small affected area for the first 3 days. If no discomfort occurs, follow directions above.
Inactive ingredients
aloe barbadensis leaf juice, ascorbic acid, astragalus membranaceus root extract, butylene glycol, butyl methoxydibenzoylmethane, caprylyl glycol, cucumis sativus (cucumber) fruit extract, DMDM hydantoin, edetate disodium, ethylhexyl methoxycinnamate, ethylhexyl salicylate, forsythia suspensa fruit extract, fragrance, green 3, iodopropynyl butylcarbamate, lactic acid, lauramidopropyl betaine, lauryl glucoside, menthol, octyldodecanol, PEG-8 dimethicone, PEG-150 distearate, PEG-40 hydrogenated castor oil, phenoxyethanol, polysorbate 20, PPG-26-buteth-26, purified water, retinyl palmitate, silica, sodium C14-16 olefin sulfonate, sodium chloride, sodium propoxyhydroxypropyl thiosulfate silica, sorbic acid, tocopheryl acetate, trolamine, yellow 5
| OXY SKIN CLEARING SOOTHING CLEANSER
DAILY DEFENSE
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |