CREST PRO-HEALTH CLINICAL GUM PROTECTION- stannous fluoride paste, dentifrice
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Crest
®
PRO-HEALTH
®
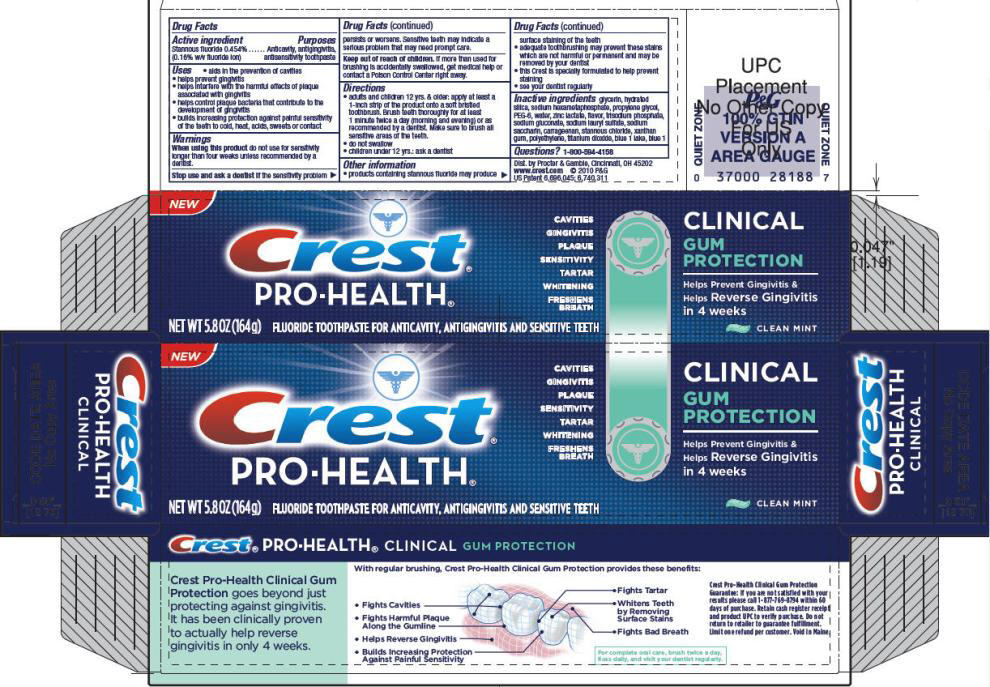
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with the harmful effects of plaque associated with gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets or contact
Warnings
When using this product do not use for sensitivity longer than four weeks unless recommended by a dentist.
Directions
- adults and children 12 yrs. & older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist. Make sure to brush all sensitive areas of the teeth.
- do not swallow
- children under 12 yrs.: ask a dentist
Other information
- products containing stannous fluoride may produce surface staining of the teeth
- adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist
- this Crest is specially formulated to help prevent staining
- see your dentist regularly
Inactive ingredients
glycerin, hydrated silica, sodium hexametaphosphate, propylene glycol, PEG-6, water, zinc lactate, flavor, trisodium phosphate, sodium gluconate, sodium lauryl sulfate, sodium saccharin, carrageenan, stannous chloride, xanthan gum, polyethylene, titanium dioxide, blue 1 lake, blue 1
PRINCIPAL DISPLAY PANEL - 164g Tube Carton
NEW
Crest
®
PRO-HEALTH
®
CAVITIES
GINGIVITIS
PLAQUE
SENSITIVITY
TARTAR
WHITENING
FRESHENS
BREATH
NET WT 5.8 OZ (164 g)
FLUORIDE TOOTHPASTE FOR ANTICAVITY, ANTIGINGIVITIS AND SENSITIVE TEETH
CLINICAL
GUM
PROTECTION
Helps Prevent Gingivitis &
Helps Reverse Gingivitis
in 4 weeks
CLEAN MINT
| CREST PRO-HEALTH
CLINICAL GUM PROTECTION
stannous fluoride paste, dentifrice |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |