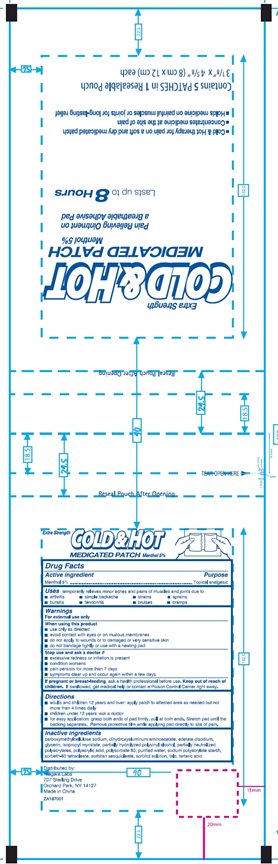
COLD AND HOT MEDICATED- menthol patch
The Mentholatum Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
temporarily relieves minor aches and pains of muscles and joints due to
- •
- arthritis
- •
- simple backache
- •
- strains
- •
- sprains
- •
- bursitis
- •
- tendonitis
- •
- bruises
- •
- cramps
Warnings
When using this product
- •
- use only as directed
- •
- avoid contact with eyes or on mucous membranes
- •
- do not apply to wounds or to damaged or very sensitive skin
- •
- do not bandage tightly or use with a heating pad
Directions
- •
- adults and children 12 years and over: apply patch to affected area as needed but not more than 4 times daily
- •
- children under 12 years: ask a doctor
- •
- for easy application; grasp both ends of pad firmly, pull at both ends. Stretch pad until the backing separates. Remove protective film while applying pad directly to site of pain.
Inactive ingredients
carboxymethylcellulose sodium, dihydroxyaluminum aminoacetate, edetate disodium, glycerin, isopropyl myristate, partially hydrolyzed polyvinyl alcohol, partially neutralized polyacrylates, polyacrylic acid, polysorbate 80, purified water, sodium polyacrylate starch, sorbeth-60 tetraoleate, sorbitan sesquioleate, sorbitol solution, talc, tartaric acid
| COLD AND HOT MEDICATED
menthol patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - The Mentholatum Company (002105757) |
Revised: 11/2016
Document Id: ed396d76-3551-4c04-8e93-cbd70e3c8346
Set id: 09c95d9b-aec9-47d2-93b4-1a33b44dd0ff
Version: 2
Effective Time: 20161104
The Mentholatum Company