DONATUSS DC- dihydrocodeine bitartrate, phenylephrine hydrochloride, guaifenesin syrup
Laser Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Donatuss DC Syrup
Rx Only
CIII
Description
Each 5 mL (one teaspoonful) for oral administration contains:
Dihydrocodeine Bitartrate ..................... 7.5 mg
(WARNING- May be habit forming)
Phenylephrine Hydrochloride................... 7.5 mg
Guaifenesin........................................... 50 mg
This product contains the following inactive ingredients: Bitter Mask, FD and C Blue #1, FD and C Red #40,
Grape Flavor, Propylene Glycol, Purified Water, Sodium Saccharin, Sucrose.
This product contains ingredients of the following therapeutic classes: Antitussive, Decongestant and
Expectorant.
Dihydrocodeine Bitartrate is an antitussive with the chemical name (Morphinan-6-ol,4,5-epoxy-3-methoxy-
17-methyl-,(5α, 6α)-2,3-dihydroxybutanedioate (1:1) (salt). It has the following structural formula:
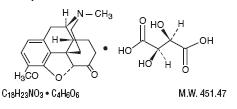
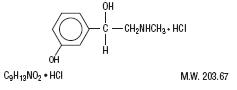
Phenylephrine hydrochloride is an orally effective nasal decongestant. Chemically it is Benzenemethanol,
3-hydroxy-α-[(methylamino)methyl]-, hydrochloride (R)-(-)-m-Hydroxy-α- [(methylamino)methyl]benzyl alcohol
hydrochloride. Its chemical structure is as follows:
Guaifenesin is an expectorant with the chemical name 1,2-Propanediol,3-(2-methoxyphenoxy)-, (±)-. It has the
following structural formula:
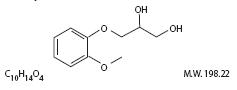
CLINICAL PHARMACOLOGY
Dihydrocodeine is a semisynthetic narcotic analgesic/antitussive related to codeine, with multiple actions
qualitatively similar to those of codeine: the most prominent of these involve the central nervous system
and organs with smooth muscle components.
Phenylephrine hydrochloride affects its vasoconstrictor activity by releasing noradrenaline from sympathetic
nerve endings, and from direct stimulation of α-adrenoreceptos in blood vessels.
Guaifenesin is an expectorant, which increases respiratory tract fluid secretions and helps to loosen phlegm
and bronchial secretions. By reducing the viscosity of secretions, guaifenesin increases the efficiency of the
cough reflex and of ciliary action in removing accumulated secretions from the trachea and bronchi.
INDICATIONS AND USAGE
This product is indicated for the temporary relief of nasal congestion and dry, non-productive cough associated
with upper respiratory tract infections and allergies.
CONTRAINDICATIONS
This combination product is contraindicated in patients with hypersensitivity to dihydrocodeine, codeine,
or any of the active or inactive components listed above, or in any situation where opioids are contraindicated
including significant respiratory depression (in unmonitored settings or in the absence of resuscitation
equipment), acute or severe bronchial asthma or hypercapnia, and paralytic ileus. Sympathomimetic agents
are contraindicated in patients with severe hypertension, severe coronary artery disease, patients with narrow
angle glaucoma, bronchial asthma, urinary retention, peptic ulcer, and during an asthmatic attack. This product
is contraindicated in women who are pregnant.
WARNINGS
General: Considerable caution should be exercised in patients with hypertension, diabetes
mellitus, ischemic heart disease, hyperthyroidism, increased intraocular pressure, and prostatic
hypertrophy. The elderly (60 years and older) are more likely to exhibit adverse reactions.
Usage in Ambulatory Patients: Dihydrocodeine may impair the mental and/or physical abilities
required for the performance of potentially hazardous tasks such as driving a car or operating
machinery.
Respiratory Depression: Respiratory depression is the most dangerous acute reaction produced
by opioid agonist preparations, although it is rarely severe with usual doses. Opioids decrease the
respiratory tidal volume, minute ventilation, and sensitivity to carbon dioxide. Respiratory
depression occurs most frequently in elderly or debilitated patients, usually after large initial doses
in non-tolerant patients, or when opioids are given in conjunction with other agents that depress
respiration. This combination product should be used with caution in patients with significant chronic
obstructive pulmonary disease or cor pulmonale and in patients with a substantially decreased
respiratory reserve, hypoxia, hypercapnia, or respiratory depression.
Hypertensive Effect: Dihydrocodeine, like all opioid analgesics, may cause hypotension in patients
whose ability to maintain blood pressure has been compromised by a depleted blood volume or who
received concurrent therapy with drugs such as phenothiazine or other agents which compromise
vasomotor tone. This product may produce orthostatic hypotension in ambulatory patients. This
combination product should be administered with caution to patients with circulatory shock since
vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Dependence: Dihydrocodeine can produce drug dependence of the codeine type and has the potential
of being abused. This product should be prescribed and administered with the appropriate degree of caution
(See Drug Abuse and Dependence section).
PRECAUTIONS
General: This combination product should be used with caution in elderly or debiliated
patients or those with any of the following conditions: adrenocortical insufficiency (e.g.,
Addison's disease); asthma; central nervous system depression or coma; chronic obstructive
pulmonary disease; decreased respiratory reserve (including emphysema, severe obesity,
cor pulmonale, or kyphoscoliosis); delirium tremens; diabetes, head injury; hypotension;
hypertension; increased intracranial pressure; myxedema or hypothyroidism; prostatic
hypertrophy or urethral structure; and toxic psychosis. The benefits and risks of opioids in
patients taking monoamine oxidase inhibitors and in those with a history of drug abuse should
be carefully considered. This combination product may aggravate convulsions in patients with
convulsive disorders, and like all opioids, may induce or aggravate seizures in some clinical settings.
Drug Interactions:
General: Sympathomimetic amines may reduce the antihypertensive effects of methyldopa,
mecamylamine, reserpine, and veratrum alkaloids.
Other CNS Depressants: Patients receiving other opioid analgesics, sedatives or hypnotics,
muscle relaxants, general anesthetics, centrally acting anti-emetics, phenothiazines or other
tranquilizers, or alcohol concomitantly with this product may exhibit additive depressant
effects on the central nervous system. When such combination therapy is contemplated,
the dose of one or both agents should be reduced. Concomitant use of dihydrocodeine with
alcohol and other CNS depressants may have an additive effect.
Monoamine Oxidase Inhibitors: Dihydrocodeine, like all opioids, interact with monoamine oxidase
inhibitors causing central nervous system excitation and hypertension. MAO inhibitors and
beta-adrenergic blockers increase the affects of sympathomimetics.
Information for Patients:
Patients receiving this product should be given the following information:
- This product may inhibit mental pr physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Report any adverse experiences occurring during therapy.
- Do not adjust the dose of this product without consulting the prescribing professional.
- Do not combine this product with alcohol or other central nervous system depressants.
- Women of childbearing potential who become, or are planning to become pregnant should be advised to consult their physician regarding the effects of opioids and other drug use during pregnancy on themselves and their unborn child.
Patients should be advised that this product is a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
Pregnancy:
Teratogenic Effects- Pregnancy Category C: Animal reproduction studies have not been conducted with this product. It is also not known whether this combination product can cause fetal harm when administered to pregnant women or can affect reproduction capacity in males
and females. This combination product should be given to a pregnant woman only if clearly needed, especially during the first trimester.
Nonteratogenic Effects:
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of the maternal opioid use or dose. There is no consensus on the best method of managing withdrawal. Chlorpromazine 0.7-1.0 mg/kg q6h, phenobarbital 2 mg/kg q6h, and paregoric 2-4 drops/kg q6h,
have been used to treat withdrawal symptoms in infants. The duration of therapy is 4 to 28 days, with dosages decreased as tolerated.
Labor and Delivery:
This product is not recommended for use by women during and immediately before labor
and delivery because oral opioids may cause respiratory depression in the newborn.
Nursing Mothers:
Due to the possible passage of the ingredients into breast milk, this product should not
be given to nursing mothers.
Pediatric Use:
This product is not recommended for use in children under six years of age. Children under two years
may be more susceptible to respiratory arrest, coma, and death. Very young children may be more
susceptible to the effects, especially the vasopressor effects of sympathomimetic amines. Appropriate
studies on the relationship of age to the effects of guaifenesin have not been performed in the pediatric
population. However, no pediatric specific problems have been documented to date.
Geriatric Use:
This product should be given with caution to the elderly.
Hepatic Impairment: This product should be given with caution to patients with hepatic insufficiency.
Since dihydrocodeine is metabolized by the liver the effects of this combination product should be
monitored closely in such patients.
Renal Impariment: This product should be used with caution and at reduced dosage in the presence of
impaired renal function.
Pancreatic/biliary Tract Disease: Opioids may cause spasms of the spinchter of Oddi and should be
used with caution in patients with biliary tract disease, including pancreatitis.
ADVERSE REACTIONS
The most frequently observed adverse reactions with dihydrocodeine include light-headedness,
dizziness, drowsiness, headache, fatigue, sedation, sweating, nausea, vomiting, constipation,
pruritus, and skin reactions. With the exception of constipation, tolerance develops to most of
these effects. Other reactions that have been observed with dihydrocodeine or opioids include
respiratory depression, orthostatic hypotension, cough suppression, confusion, diarrhea, miosis,
abdominal pain, dry mouth, indigestion, anorexia, spasm of biliary tract, and urinary retention.
Physical and psychological dependence are possibilities. Hypersensitivity reactions (include
anaphylactoid reactions), hallucinations, vivid dreams, granulomatous interstitial nephritis, severe
narcosis and acute renal failure have been reported rarely during dihydrocodeine administration.
Other reactions observed with the ingredients of this product include lassitude, nausea, giddiness,
dryness of mouth, blurred vision, cardiac palpitations, flushing, increased irritability or excitement
(especially in children).
DRUG ABUSE AND DEPENDENCE
This combination product is subject to the provisions of the Controlled Substances Act and
has been placed in Schedule III. Dihydrocodeine can produce drug dependence of the codeine
type and therefore has the potential of being abused. Psychological dependence, physical
dependence, and tolerance may develop upon repeat administration of dihydrocodeine, and it
should be prescribed and administered with the same degree of caution appropriate to the use
of other opioid medications. Symptoms of dihydrocodeine withdrawal consist of irritability,
restlessness, insomnia, diaphoresis, anxiety, and palpitations.
OVERDOSAGE
An overdose of this product is a potentially lethal poly-drug overdose situation, and
consultation with a regional Poison Control Center is recommended. A listing of the
Poison Control Centers can be found in a standard reference such as the Physician's
Desk Reference.
Signs and Symptoms:
Symptoms of an overdose include pinpoint pupils, respiratory depression, extreme
somnolence progressing to stupor, loss of consciousness or coma, skeletal muscle
flaccidity, cold and clammy skin and other symptoms common with narcotic overdosage.
Convulsions, cardiovascular collapse, and death may occur. A single case of acute
rhabdomyolysis associated with an overdose of dihydrocodeine has been reported.
Recommended Treatment:
Immediate treatment of an overdose of this product includes support of cardiovascular
function and measure to reduce further drug absorption. Vomiting should be induced with
syrup of ipecac. If the patient is alert and has adequate laryngeal reflexes, oral activated
charcoal should follow. The first dose should be accompanied by an appropriate cathartic.
Gastric lavage may be necessary. Hypotension is usually hypovolemic and should be
treated with fluids. Endotracheal intubation and artificial respiration may be necessary.
The pure opioid antagonist naloxone or nalmexone is a specific antidote against respiratory
depression that results from opioid overdose. Opioid antagonists should not be given in the
absence of clinically significant respiratory or circulatory depression secondary to opioid
overdose. They should be administered cautiously to persons who are known, or suspected
to be, physically dependent on any opioid agonist including dihydrocodeine. In such cases,
an abrupt or complete reversal of opioid effects may precipitate an acute abstinence
syndrome. The prescribing information for the specific opioid antagonist should be consulted
for details of their proper use.
DOSAGE AND ADMINISTRATION
Adults and children over 12 years:
1 to 2 teaspoonfuls (5 mL to 10 mL) every 4 to 6 hours, as needed.
Children 6 to under 12 years of age:
1/2 to 1 teaspoonful (2.5 mL to 5 mL), every 4 to 6 hours, as needed.
Not recommended for children under 6 years of age.
HOW SUPPLIED
Donatuss DC Syrup is supplied as a alcohol free, gluten free syrup with a grape flavor
in 16 fl oz (473 mL) bottles, NDC 16477-136-16, and 15 mL professional samples,
NDC 16477-136-15.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE
OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT
A POISON CONTROL CENTER IMMEDIATELY.
Store at controlled room temperature, 15o-30oC (59o-86oF).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container as defined in the USP/NF with a
child-resistant closure. (This statement is only on the 16 oz. Label)
This bottle is not to be dispensed to consumer. (This statement is only on the 16 oz label)
Supplied in a tight, light-resistant container as defined in the USP/NF with a child-resistant
closure. (This statement is only on the 15 mL Label)
RX Only
Manufactured for:
Laser Pharmaceuticals, Inc.
Greenville, SC 29615
Manufactured by:
Great Southern Laboratories
Houston, TX 77099
Rev. 09/09
PRODUCT PACKAGING
The packaging below represents the labeling currently used.
Principal Display Panel and Side Panel for 16 oz. (473 mL) Label:
NDC 16477-136-16
Donatuss DC
SYRUP
Rx
Antitussive
Decongestant
Expectorant
Each 5 mL (1 teaspoonful) contains:
Dihydrocodeine Bitartrate* ... 7.5 mg
(WARNING: May be habit-forming.)
Phenylephrine HCl ............. 7.5 mg
Guaifenesin ....................... 50 mg
Alcohol Free / Gluten Free

Rx Only 16 fl oz (473 mL)
DOSAGE AND ADMINISTRATION:
Adults and children over 12 years:
1 to 2 teaspoonfuls (5 mL to 10 mL), every 4 to 6 hours, as needed.
Children 6 to under 12 years of age:
1/2 to 1 teaspoonful (2.5 mL to 5 mL), every 4 to 6 hours, as needed.
Not recommended for children under 6 years of age.
Store at controlled room temperature 15o-30oC (59o-86oF).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
This bottle is not to be dispensed to consumer.
Pharmacist: Dispense in a tight, light-resistant container with a child-resistant
closure as defined in the USP/NF.
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH
OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK
PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL
CENTER IMMEDIATELY.

Manufactured for: Manufactured by:
Laser Pharmaceuticals, LLC Great Southern Laboratories
Greenville, SC 29615 Houston, TX 77099
Rev. 09/09
Principal Display Panel and Side Panel for 15 mL Label:
NDC: 16477-136-15
Donatuss DC
Syrup
Rx
Antitussive
Decongestant
Expectorant
Each 5 mL (1 teaspoonful) contains:
Dihydrocodeine Bitartrate* .. 7.5 mg
(*Warning: May be habit forming)
Phenylephrine HCl ............. 7.5 mg
Guaifenesin........................ 50 mg

Rx Only 15 mL (1/2 fl oz)
See product foldout for full prescribing information.
Store at controlled room temperature, 15o-30oC (59o-86oF).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container as defined in the USP/NF with a
child-resistant closure.
WARNING: Keep this and all drugs out of the reach of children.
Manufactured for:
Laser Pharmaceuticals, LLC
Greenville, SC 29615
Manufactured by:
Great Southern Laboratories
Houston, TX 77099 Rev. 09/09
Professional Sample: Not For Sale





| DONATUSS
DC
dihydrocodeine bitartrate, phenylephrine hydrochloride, guaifenesin syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Laser Pharmaceuticals, LLC (614417132) |
| Registrant - Great Southern Laboratories (056139553) |