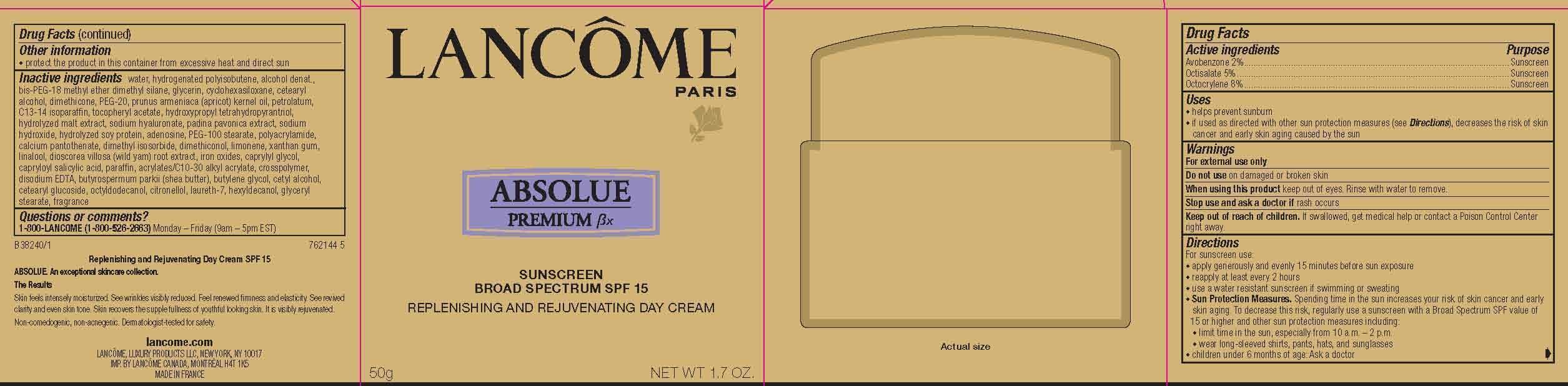
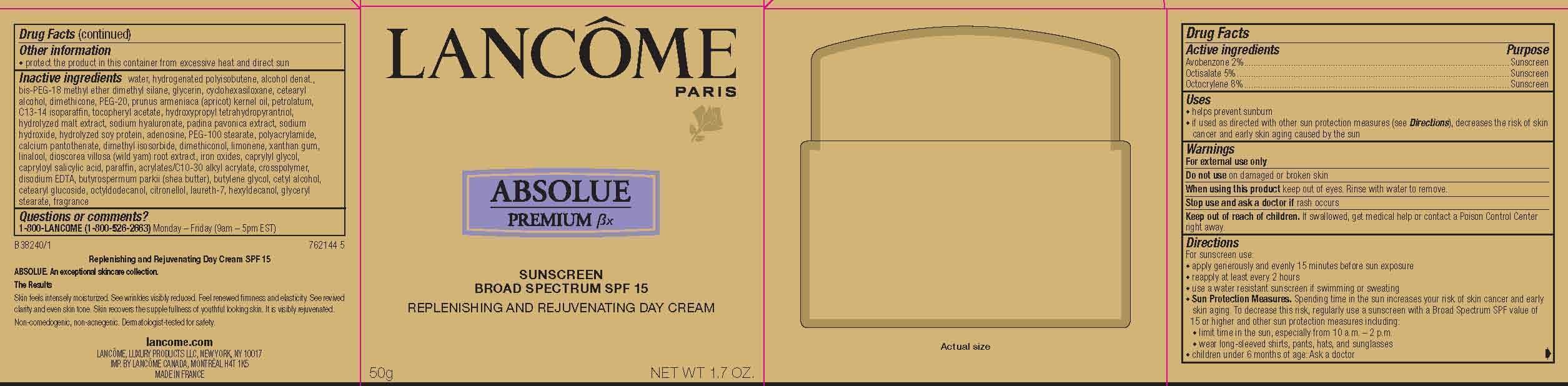
Label: LANCOME PARIS ABSOLUE PREMIUM BX REPLENISHING AND REJUVENATING DAY- avobenzone, octisalate and octocrylene cream
- NDC Code(s): 49967-952-01, 49967-952-02, 49967-952-03, 49967-952-04
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
● apply generously and evenly 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, hydrogenated polyisobutene, alcohol denat., bis-PEG-18 methyl ether dimethyl silane, glycerin, cyclohexasiloxane, cetearyl alcohol, dimethicone, PEG-20, prunus armeniaca (apricot) kernel oil, petrolatum, C13-14 isoparaffin, tocopheryl acetate, hydroxypropyl tetrahydropyrantriol, hydrolyzed malt extract, sodium hyaluronate, padina pavonica extract, sodium hydroxide, hydrolyzed soy protein, adenosine, PEG-100 stearate, polyacrylamide, calcium pantothenate, dimethyl isosorbide, dimethiconol, limonene, xanthan gum, linalool, dioscorea villosa (wild yam) root extract, iron oxides, caprylyl glycol, capryloyl salicylic acid, paraffin, acrylates/C10-30 alkyl acrylate, crosspolymer, disodium EDTA, butyrospermum parkii (shea butter), butylene glycol, cetyl alcohol, cetearyl glucoside, octyldodecanol, citronellol, laureth-7, hexyldecanol, glyceryl stearate, fragrance
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANCOME PARIS ABSOLUE PREMIUM BX REPLENISHING AND REJUVENATING DAY
avobenzone, octisalate and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-952 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 20 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 80 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-952-01 1 in 1 CARTON 06/01/2010 1 50 g in 1 JAR; Type 0: Not a Combination Product 2 NDC:49967-952-02 1 in 1 CARTON 06/01/2010 2 75 g in 1 JAR; Type 0: Not a Combination Product 3 NDC:49967-952-03 1 in 1 CARTON 06/01/2010 3 15 g in 1 JAR; Type 0: Not a Combination Product 4 NDC:49967-952-04 2 g in 1 PACKET; Type 0: Not a Combination Product 06/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2010 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations SICOS ET CIE 276993581 manufacture(49967-952) , pack(49967-952) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 185931458 manufacture(49967-952) , pack(49967-952)