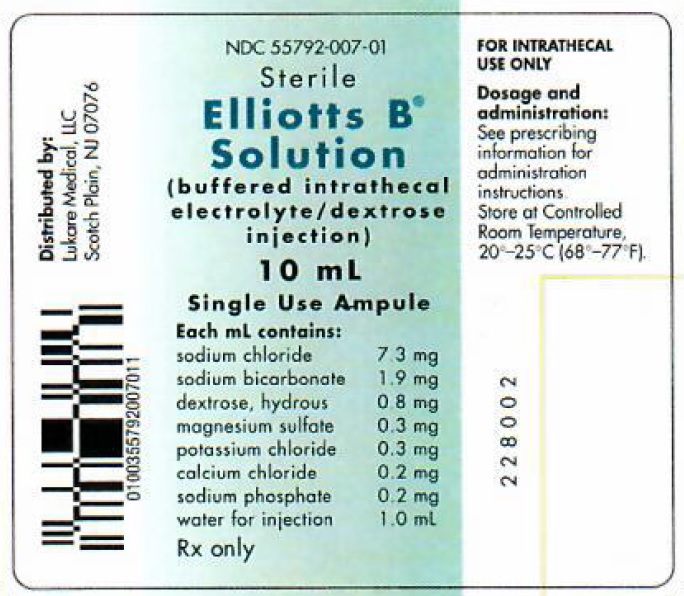
Label: ELLIOTTS B- sodium cation, sodium bicarbonate, anhydrous dextrose, magnesium sulfate, potassium chloride, calcium chloride, sodium phosphate injection
- NDC Code(s): 55792-007-01, 55792-007-10
- Packager: Lukare Medical, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 16, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Elliotts B® Solution is a sterile, nonpyrogenic, isotonic solution containing no bacteriostatic preservatives. Elliotts B Solution is a diluent for intrathecal administration of methotrexate sodium and cytarabine.
Each 10 mL of Elliotts B Solution contains:
Sodium Chloride, USP 73 mg Sodium Bicarbonate, USP 19 mg Dextrose, USP 8 mg Magnesium Sulfate • 7H2O, USP 3 mg Potassium Chloride, USP 3 mg Calcium Chloride • 2H2O, USP 2 mg Sodium Phosphate, dibasic • 7H2O, USP 2 mg Water for Injection, USP qs 10 mL
Concentration of Electrolytes:Sodium 149 mEq/liter Bicarbonate 22.6 mEq/liter Potassium 4.0 mEq/liter Chloride 132 mEq/liter Calcium 2.7 mEq/liter Sulfate 2.4 mEq/liter Magnesium 2.4 mEq/liter Phosphate 1.5 mEq/liter The formulae and molecular weights of the ingredients are:
INGREDIENT MOLECULAR
FORMULAMOLECULAR
WEIGHTSodium Chloride NaCl 58.44 Sodium Bicarbonate NaHCO3 84.01 Dextrose C6H12O6 180.16 Magnesium Sulfate • 7H2O Mg2SO4 • 7H2O 246.48 Potassium Chloride KCl 74.55 Calcium Chloride • 2H2O CaCl2 • 2H2O 147.01 Sodium Phosphate, dibasic • 7H2O Na2HPO4 • 7H2O 268.07
The pH of Elliotts B Solution is 6.0-7.5, and the osmolarity is 288 mOsmol per liter (calculated). -
CLINICAL PHARMACOLOGY
Elliotts B Solution provides a buffered salt solution for use as a diluent for the intrathecal administration of methotrexate sodium and cytarabine. It has been demonstrated that Elliotts B Solution is comparable to cerebrospinal fluid in pH, electrolyte composition, glucose content, and osmolarity:
Comparison of Electrolyte Composition, pH and Nonelectrolytic Constituents of Elliotts B Solution and CSF
Solution
Na+
mEq/LK+
mEq/LCa++
mEq/LMg++
mEq/LHCO3-
mEq/LCl-
mEq/LpH
Phosphorus
mg/dLGlucose
mg/dLCerebrospinal Fluid
117-137
2.3-4.6
2.2
2.2
22.9
113-127
7.31
1.2-2.1
45-80
Elliotts B Solution
149
4.0
2.7
2.4
22.6
132
6.0-7.5
2.3
80
The approximate buffer capacity of Elliotts B Solution is 1.1 X 10-2 equivalents when the challenge solution is 0.01 N HCl and 7.8 X 10-3 equivalents when the challenge solution is 0.01 N NaOH.1
Compatibility studies with methotrexate sodium and cytarabine indicate these drugs are physically compatible with Elliotts B Solution.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Intrathecal administration of drugs such as methotrexate sodium and cytarabine should be performed by personnel skilled in the technique of lumbar puncture under the supervision of a physician who is experienced in the use of cancer chemotherapeutic agents. The labeling for methotrexate sodium and cytarabine should be consulted.
-
PRECAUTIONS
General
Particular attention should be taken to assure the maintenance of sterile technique throughout the procedure. (See DOSAGE AND ADMINISTRATION.)
-
ADVERSE REACTIONS
Adverse reactions may occur with any given intrathecal injection due to the chemotherapy or the technique of intrathecal administration. (See product labeling for methotrexate sodium and cytarabine.)
Preservative-free methotrexate sodium and cytarabine should be used to minimize adverse reactions due to preservatives.
If an adverse reaction does occur, discontinue the administration, evaluate the patient, institute appropriate therapeutic countermeasures and, if possible, save the remainder of the unused solution(s) for examination.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
See product labeling for methotrexate sodium and cytarabine.
Elliotts B Solution is intended for intrathecal administration only. Elliotts B Solution does not contain antibacterial preservatives and introduction of contaminated solutions into the cerebrospinal fluid may have extremely serious consequences. Therefore, administration of intrathecal solutions should be accomplished as soon as possible after preparation.
A sterile filter-needle should be used to withdraw the contents of the ampule.
Intrathecal drug products should be inspected visually for particulate matter and discoloration prior to administration.
Preparation and Administration Precautions
Elliotts B Solution is a diluent for the cytotoxic anticancer agents, methotrexate sodium and cytarabine. Care should be exercised in the handling and preparation of infusion solutions with these products. (See product labeling for methotrexate sodium and cytarabine.)
-
HOW SUPPLIED
NDC SIZE 55792-007-10 10 mL ampule Elliotts B Solution is available in single-use clear glass ampules, packaged 10 ampules per box.
Store at controlled room temperature, 20º-25ºC (68º-77ºF) [See USP].
Preservative Free. Discard unused portion. Use only if solution is clear and ampule is intact.
Distributed by:
Lukare Medical, LLC
Scotch Plains, NJ 07076
1-855-752-9317
www.elliottsbsolution.com - REFERENCES:
- PACKAGING
-
INGREDIENTS AND APPEARANCE
ELLIOTTS B
sodium cation, sodium bicarbonate, anhydrous dextrose, magnesium sulfate, potassium chloride, calcium chloride, sodium phosphate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55792-007 Route of Administration INTRATHECAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium chloride (UNII: 451W47IQ8X) (sodium cation - UNII:LYR4M0NH37, chloride ion - UNII:Q32ZN48698) sodium chloride 73 mg in 10 mL sodium bicarbonate (UNII: 8MDF5V39QO) (bicarbonate ion - UNII:HN1ZRA3Q20) sodium bicarbonate 19 mg in 10 mL anhydrous dextrose (UNII: 5SL0G7R0OK) (anhydrous dextrose - UNII:5SL0G7R0OK) anhydrous dextrose 8 mg in 10 mL MAGNESIUM SULFATE, UNSPECIFIED (UNII: DE08037SAB) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE, UNSPECIFIED 3 mg in 10 mL potassium chloride (UNII: 660YQ98I10) (potassium cation - UNII:295O53K152) potassium chloride 3 mg in 10 mL calcium chloride (UNII: M4I0D6VV5M) (calcium cation - UNII:2M83C4R6ZB) calcium chloride 2 mg in 10 mL sodium phosphate (UNII: SE337SVY37) (phosphate ion - UNII:NK08V8K8HR) sodium phosphate 2 mg in 10 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55792-007-10 10 in 1 BOX 06/04/2013 1 NDC:55792-007-01 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020577 06/04/2013 Labeler - Lukare Medical, LLC (062862393) Registrant - Lukare Medical, LLC (062862393)