ACNEFREE CLEAR SKIN TREATMENTS 24 HOUR SEVERE ACNE CLEARING SYSTEM- benzoyl peroxide
Bausch Health US, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
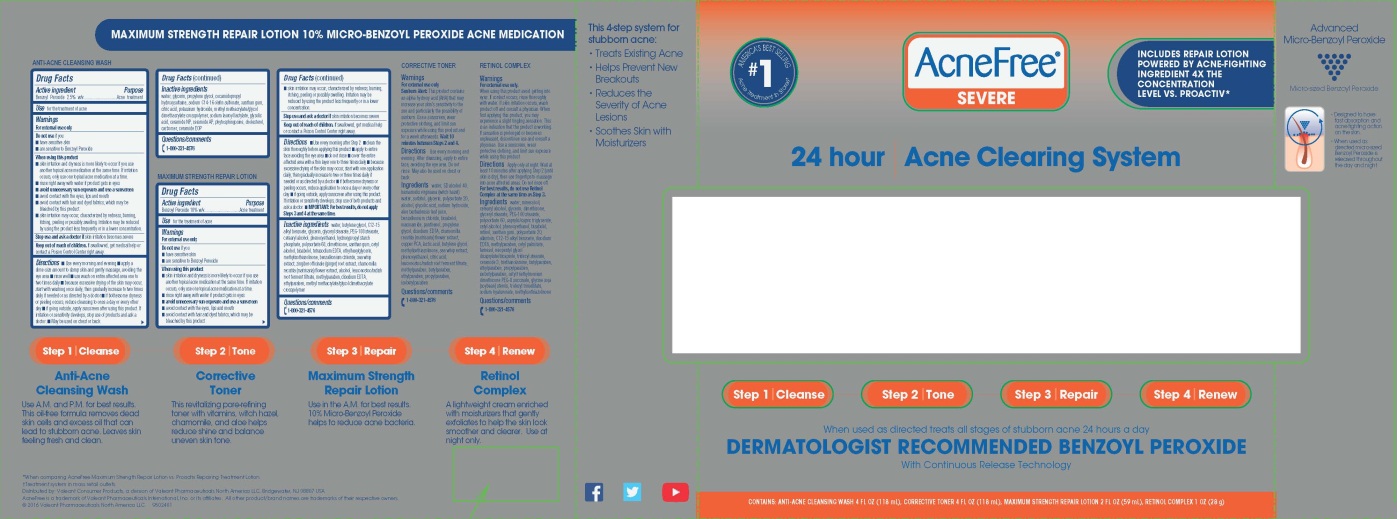
AcneFree® Severe 24 hour Severe Acne Clearing System
Warnings
For external use only
When using this product
- •
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- •
- rinse right away with water if product gets in eyes
- •
- avoid unnecessary sun exposure and use a sunscreen
- •
- avoid contact with the eyes, lips and mouth
- •
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- •
- skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
Directions
- •
- Use every morning and evening.
- •
- apply a dime-size amount to damp skin and gently massage, avoiding the eye area.
- •
- rinse well
- •
- use wash on entire affected area one to two times daily
- •
- because excessive drying of the skin my occur, start with washing once daily, then gradually increase to two times daily if needed or as directed by a doctor
- •
- if bothersome dryness or peeling occurs, reduce cleansing to once a day or every other day
- •
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of products and ask a doctor.
- •
- May be used on chest or back.
Inactive ingredients
water, glycerin, propylene glycol, cocamidopropyl hydroxysultaine, sodium C14-16 olefin sulfonate, xanthan gum, citric acid, potassium hydroxide, methyl methacrylate/glycol dimethacrylate crosspolymer, sodium lauroyl lactylate, glycolic acid, ceramide NP, ceramide AP, phytosphingosine, cholesterol, carbomer, ceramide EOP
24 HOUR Severe Acne Clearing System CORRECTIVE TONER
Warnings
For external use only
Sunburn Alert: This product contains an alpha hydroxy acid (AHA) that may increase your skin’s sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product and for a week afterwards. Wait 10 minutes between Steps 2 and 4.
Directions
Use every morning and evening. After cleansing, apply to entire face, avoiding the eye area. Do not rinse. May also be used on chest or back.
Ingredients
water, SD alcohol 40, hamamelis virginiana (witch hazel) water, sorbitol, glycerin, polysorbate 20, alcohol, glycolic acid, sodium hydroxide, aloe barbadensis leaf juice, benzalkonium chloride, bisabolol, niacinamide, panthenol, propylene glycol, disodium EDTA, chamomilla recutita (matricaria) flower extract, copper PCA, lactic acid, butylene glycol, methylisothiazolinone, sea whip extract, phenoxyethanol, citric acid, leuconostoc/radish root ferment filtrate, methylparaben, butylparaben, ethylparaben, propylparaben, isobutylparaben
24 HOUR Acne Clearing System RETINOL COMPLEX
Warnings
For external use only.
When using this product avoid getting into eyes. If contact occurs, rinse thoroughly with water. If skin irritation occurs, wash product off and consult physician. When first applying this product, you may experience a slight tingling sensation. This is an indication that the product is working. If sensation is prolonged or becomes unpleasant, discontinue use and consult a physician. Use a sunscreen, wear protective clothing, and limit sun exposure while using this product.
Directions
Apply only at night. Wait at least 10 minutes after applying Step 2 (until skin is dry), then use fingertips to massage into acne affected areas. Do not rinse off. For best results, do not use Retinol Complex at the same time as Step 3.
Ingredients
water, mineral oil, cetearyl alcohol, glycerin, dimethicone, glyceryl stearate, PEG-100 stearate, polysorbate 60, caprylic/capric triglyceride, cetyl alcohol, phenoxyethanol, bisabolol, retinol, xanthan gum, polysorbate 20, allantoin, C12-15 alkyl benzoate, disodium EDTA, methylparaben, cetyl palmitate, famesol, neopentyl glycol dicaprylate/dicaprate, tridecyl stearate, ceramide 3, triethanolamine, butylparaben, ethylparaben, propylparaben, isobutylparaben, cetyl triethylmonium dimethicone PEG-8 succinate, glycine sola (soybean) sterols, tridecyl trimellitate, sodium hyaluronate, methylisothiazolinone
Warnings
For external use only
Do not use if you
- •
- have very sensitive skin
- •
- are sensitive to Benzoyl Peroxide
When using this product
- •
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- •
- rinse right away with water if product gets in eyes
- •
- avoid unnecessary sun exposure and use a sunscreen
- •
- avoid contact with the eyes, lips and mouth
- •
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- •
- skin irritation may occur, characterized by redness, burning, itching, peeling or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration..
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- Use every morning after Step 2
- •
- clean skin thoroughly before applying this product
- •
- apply to entire face avoiding the eye area
- •
- do not rinse
- •
- cover the entire affected area with a thin layer one to three times daily
- •
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- •
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- •
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- •
- IMPORTANT: For best results, do not apply Steps 3 & 4 at the same time.
Inactive ingredients
water, butylene glycol, C12-15 alkyl benzoate, glycerin, glyceryl stearate, PEG-100 stearate, cetearyl alcohol, phenoxyethanol, hydroxypropyl starch phosphate, polysorbate 60, dimethicone, xanthan gum, cetyl alcohol, bisabolol, tetrasodium EDTA, ethylhexylglycerin, methylisothiazolinone, benzalkonium chloride, sea whip extract, zingiber officinale (ginger) root extract, chamomilla recutita (matricaria) flower extract, alcohol, leuconostoc/radish root ferment filtrate, methylparaben, disodium EDTA, ethylparaben, methyl methacrylate/glycol dimethacrylate crosspolymer
PRINCIPAL DISPLAY PANEL - Kit Carton Label
AcneFree®
SEVERE
24 HOUR
Severe Acne Clearing System
AMERICA’S BEST SELLING
#1
Acne Treatment In Stores†
INCLUDES REPAIR LOTION POWERED BY ACNE-FIGHTING INGREDIENT 4XTHE CONCENTRATION LEVEL VS. PROACTIV*
When used as directed treats all stages of stubborn acne 24 hours a day
DERMATOLOGIST RECOMMENDED BENZOYL PEROXIDE
With Continuous Release Technology
CONTAINS: ANTI-ACNE CLEANSING WASH 4 FL OZ (118 mL), CORRECTIVE TONER 4 FL OZ (118 mL), MAXIMUM STRENGTH REPAIR LOTION 2 FL OZ (59 mL), RETINOL COMPLEX 1 OZ (28 g)
| ACNEFREE CLEAR SKIN TREATMENTS
24 HOUR SEVERE ACNE CLEARING SYSTEM
benzoyl peroxide kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Bausch Health US, LLC (831922468) |