Label: BE SWEET SPF 15 TINTED ABLAZE- avobenzone, octinoxate gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 72112-004-01 - Packager: Bona Fide Beauty Lab PS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 16, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
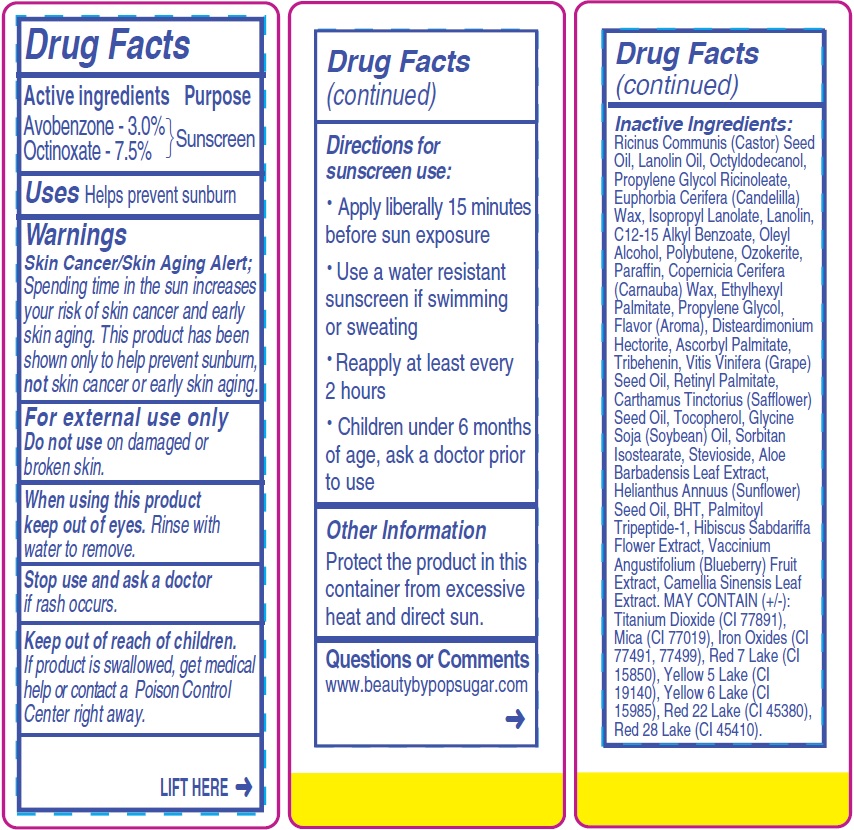
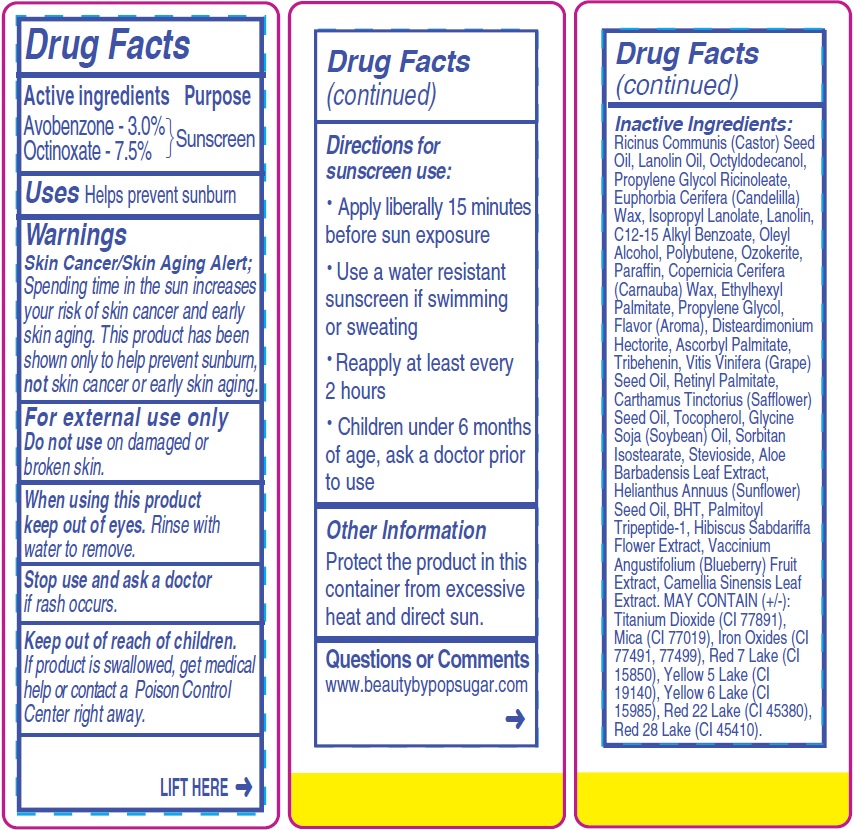
- Drug Facts
- Active ingredients
- Uses
- Warnings
- Directions for sunscreen use:
- Other Information
- Questions or Comments
-
Inactive Ingredients:
Ricinus Communis (Castor) Seed Oil, Lanolin Oil, Octyldodecanol, Propylene Glycol Ricinoleate, Euphorbia Cerifera (Candelilla) Wax, Isopropyl Lanolate, Lanolin, C12-15 Alkyl Benzoate, Oleyl Alcohol, Polybutene, Ozokerite, Paraffin, Copernicia Cerifera (Carnauba) Wax, Ethylhexyl Palmitate, Propylene Glycol, Flavor (Aroma), Disteardimonium Hectorite, Ascorbyl Palmitate, Tribehenin, Vitis Vinifera (Grape) Seed Oil, Retinyl Palmitate, Carthamus Tinctorius (Safflower) Seed Oil, Tocopherol, Glycine Soja (Soybean) Oil, Sorbitan Isostearate, Stevioside, Aloe Barbadensis Leaf Extract, Helianthus Annuus (Sunflower) Seed Oil, BHT, Palmitoyl Tripeptide-1, Hibiscus Sabdariffa Flower Extract, Vaccinium Angustifolium (Blueberry) Fruit Extract, Camellia Sinensis Leaf Extract. MAY CONTAIN (+/-): Titanium Dioxide (CI 77891), Mica (CI 77019), Iron Oxides (CI
77491, 77499), Red 7 Lake (CI 15850), Yellow 5 Lake (CI 19140), Yellow 6 Lake (CI 15985), Red 22 Lake (CI 45380), Red 28 Lake (CI 45410). - Package Labeling:
-
INGREDIENTS AND APPEARANCE
BE SWEET SPF 15 TINTED ABLAZE
avobenzone, octinoxate gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72112-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) LANOLIN OIL (UNII: OVV5IIJ58F) OCTYLDODECANOL (UNII: 461N1O614Y) PROPYLENE GLYCOL RICINOLEATE (UNII: WD6G36K42U) CANDELILLA WAX (UNII: WL0328HX19) LANOLIN (UNII: 7EV65EAW6H) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) OLEYL ALCOHOL (UNII: 172F2WN8DV) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) CERESIN (UNII: Q1LS2UJO3A) PARAFFIN (UNII: I9O0E3H2ZE) CARNAUBA WAX (UNII: R12CBM0EIZ) ETHYLHEXYL PALMITATE (UNII: 2865993309) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYL SALICYLATE (UNII: LAV5U5022Y) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ASCORBYL PALMITATE (UNII: QN83US2B0N) TRIBEHENIN (UNII: 8OC9U7TQZ0) GRAPE SEED OIL (UNII: 930MLC8XGG) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) SAFFLOWER OIL (UNII: 65UEH262IS) TOCOPHEROL (UNII: R0ZB2556P8) SOYBEAN OIL (UNII: 241ATL177A) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) STEVIOSIDE (UNII: 0YON5MXJ9P) ALOE VERA LEAF (UNII: ZY81Z83H0X) SUNFLOWER OIL (UNII: 3W1JG795YI) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HIBISCUS SABDARIFFA FLOWER (UNII: 45TGG6IU6M) LOWBUSH BLUEBERRY (UNII: G90PX41VP0) GREEN TEA LEAF (UNII: W2ZU1RY8B0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72112-004-01 1 in 1 CARTON 03/01/2018 1 2.7 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/01/2018 Labeler - Bona Fide Beauty Lab PS LLC (064526749)