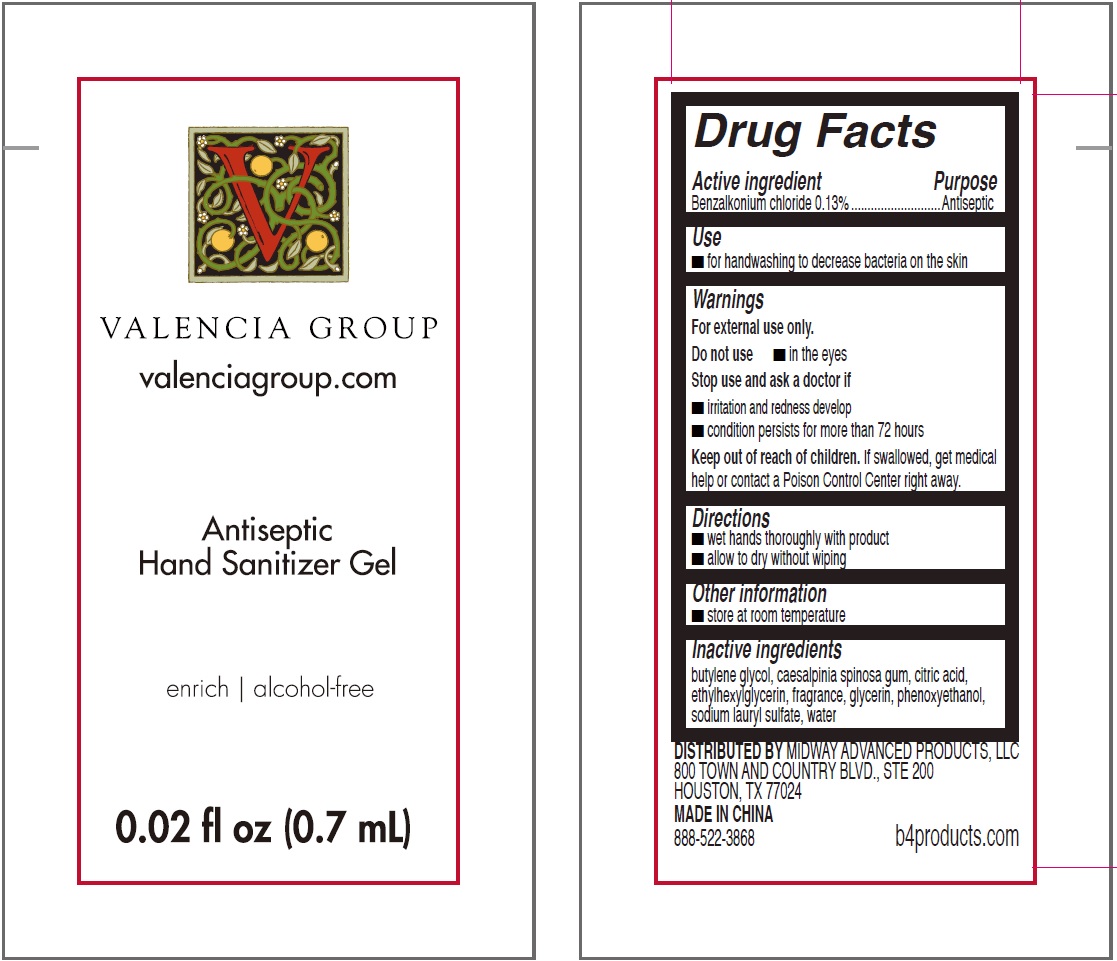
ANTISEPTIC HAND SANITIZER ALCOHOL FREE VALENCIA GROUP- benzalkonium chloride gel
Midway Advanced Products, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Antiseptic Hand Sanitizer Alcohol Free Valencia Group
Warnings
For external use only
| ANTISEPTIC HAND SANITIZER ALCOHOL FREE VALENCIA GROUP
benzalkonium chloride gel |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Midway Advanced Products, LLC (962765009) |
Revised: 7/2018
Document Id: 70ba379a-6e2f-48c1-e053-2991aa0ae4f5
Set id: 04893caa-24d4-488d-bea8-974d8dc80bd3
Version: 2
Effective Time: 20180711
Midway Advanced Products, LLC