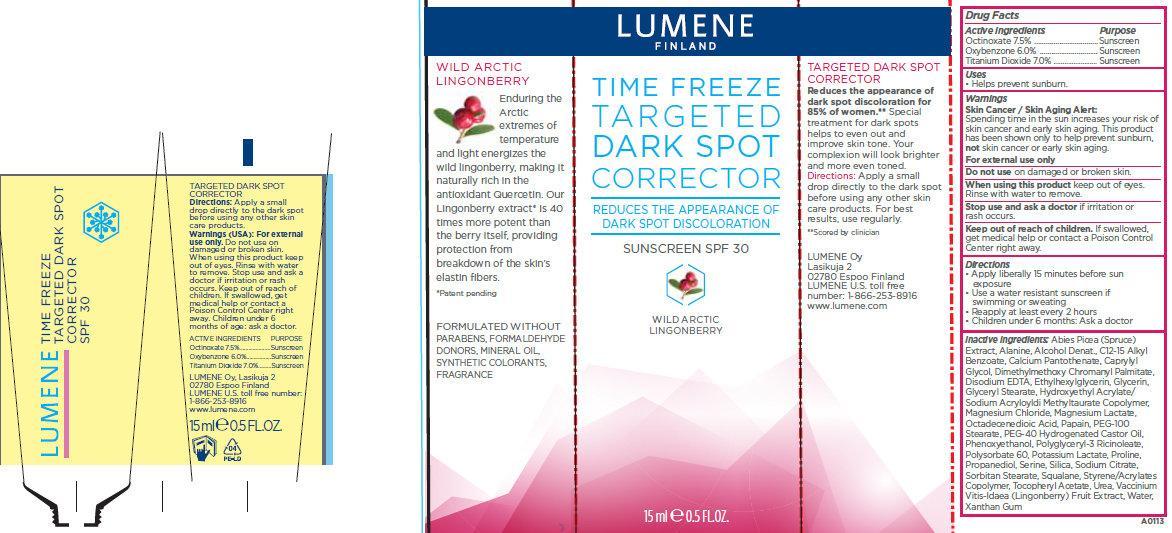
LUMENE TIME FREEZE TARGETED DARK SPOT CORRECTOR SUNSCREEN SPF 30- octinoxate, oxybenzone, titanium dioxide cream
LUMENE OY
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lumene Time Freeze Targeted Dark Spot Corrector Sunscreen SPF 30
Active ingredients
Octinoxate 7.5%
Oxybenzone 6.0%
Titanium Dioxide 7.0%
Warnings
Skin Cancer / Skin Aging Alert:
Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn,
not skin cancer or early skin aging.
For external use only
Do not use
on damaged or broken skin.
When using this product
keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor
if irritation or rash occurs.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
Inactive ingredients:
Abies Picea (Spruce) Extract, Alanine, Alcohol Denat., C12-15 Alkyl Benzoate, Calcium Pantothenate, Caprylyl Glycol, Dimethylmethoxy Chromanyl Palmitate, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Hydroxyethyl Acrylate/Sodium Acryloyldi Methyltaurate Copolymer, Magnesium Chloride, Magnesium Lactate, Octadecenedioic Acid, Papain, PEG-100 Stearate, PEG-40 Hydrogenated Castor Oil, Phenoxyethanol, Polyglyceryl-3 Ricinoleate, Polysorbate 60, Potassium Lactate, Proline, Propanediol, Serine, Silica, Sodium Citrate, Sorbitan Stearate, Squalane, Styrene/Acrylates Copolymer, Tocopheryl Acetate, Urea, Vaccinium Vitis-Idaea (Lingonberry) Fruit Extract, Water, Xanthan Gum
Lumene Time Freeze Targeted Dark Spot Corrector Sunscreen SPF 30 15ml (67692-339-00)