LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 10- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 20- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 21- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 30- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 40- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 50- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 60- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 12 ROSE- octinoxate and titanium dioxide gel
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 22 ROSE- octinoxate and titanium dioxide gel
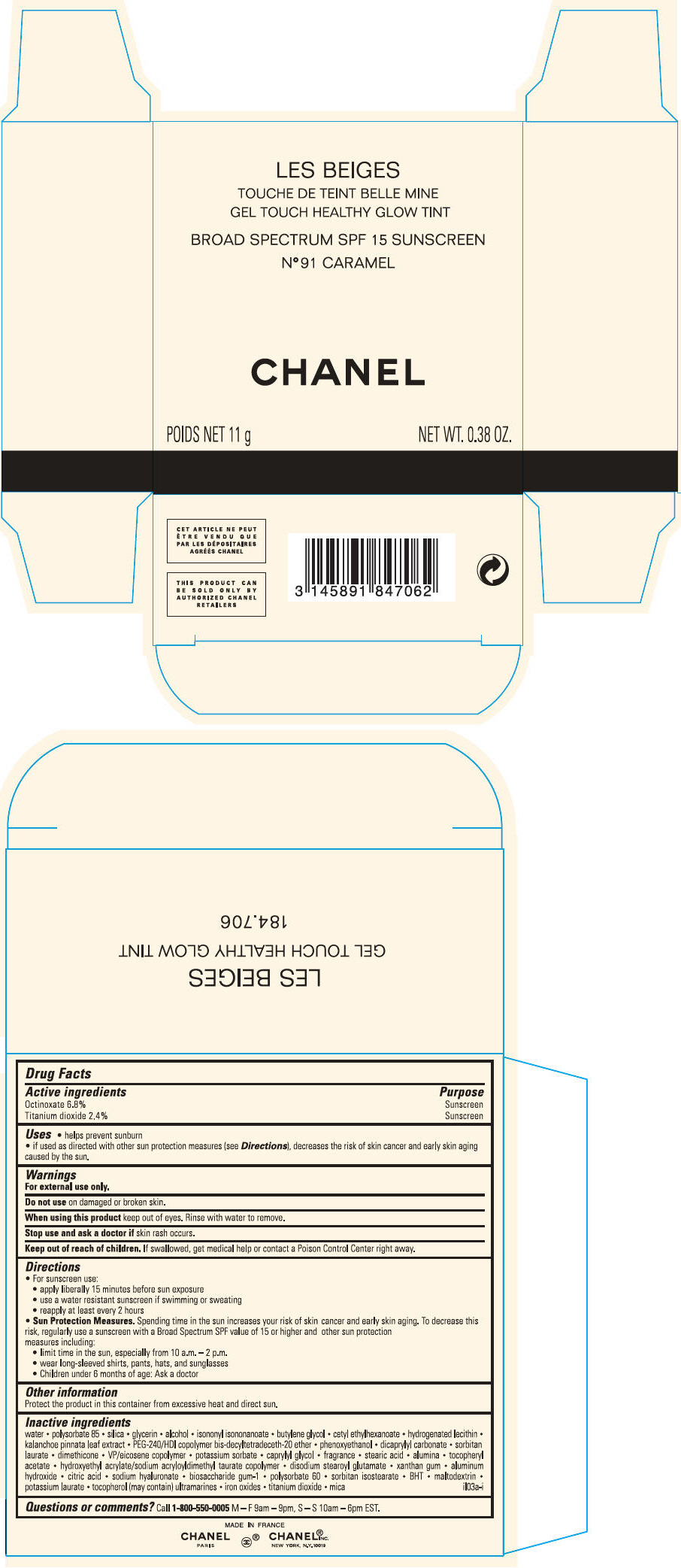
LES BEIGES GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 91 CARAMEL- octinoxate and titanium dioxide gel
CHANEL PARFUMS BEAUTE
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Les Beiges
Gel Touch Healthy Glow Tint Broad Spectrum SPF 15 Sunscreen
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
Inactive ingredients
water • polysorbate 85 • silica • glycerin • alcohol • isononyl isononanoate • butylene glycol • cetyl ethylhexanoate • hydrogenated lecithin • kalanchoe pinnata leaf extract • PEG-240/HDI copolymer bis-decyltetradeceth-20 ether • phenoxyethanol • dicaprylyl carbonate • sorbitan laurate • dimethicone • VP/eicosene copolymer • potassium sorbate • caprylyl glycol • fragrance • stearic acid • alumina • tocopheryl acetate • hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer • disodium stearoyl glutamate • xanthan gum • aluminum hydroxide • citric acid • sodium hyaluronate • biosaccharide gum-1 • polysorbate 60 • sorbitan isostearate • BHT • maltodextrin • potassium laurate • tocopherol (may contain) ultramarines • iron oxides • titanium dioxide • mica
il03a-1
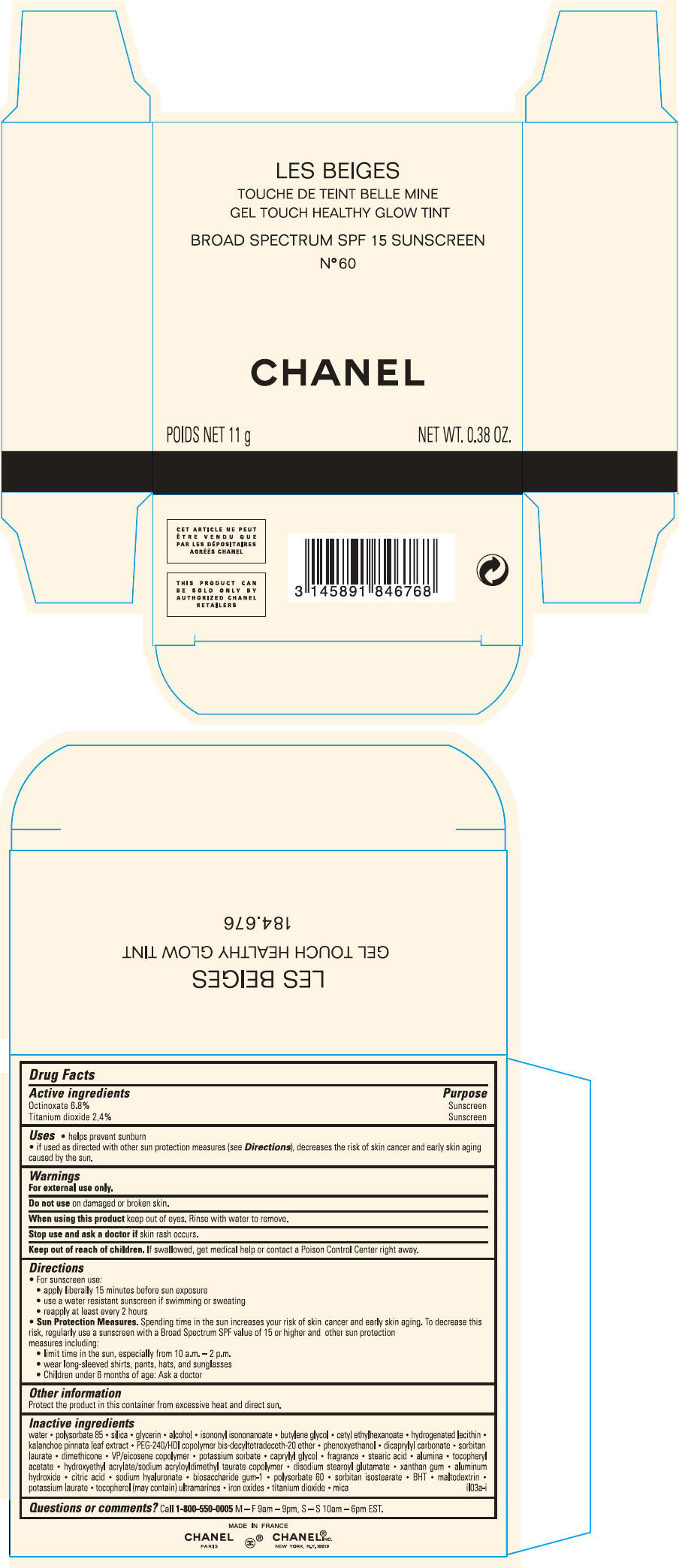
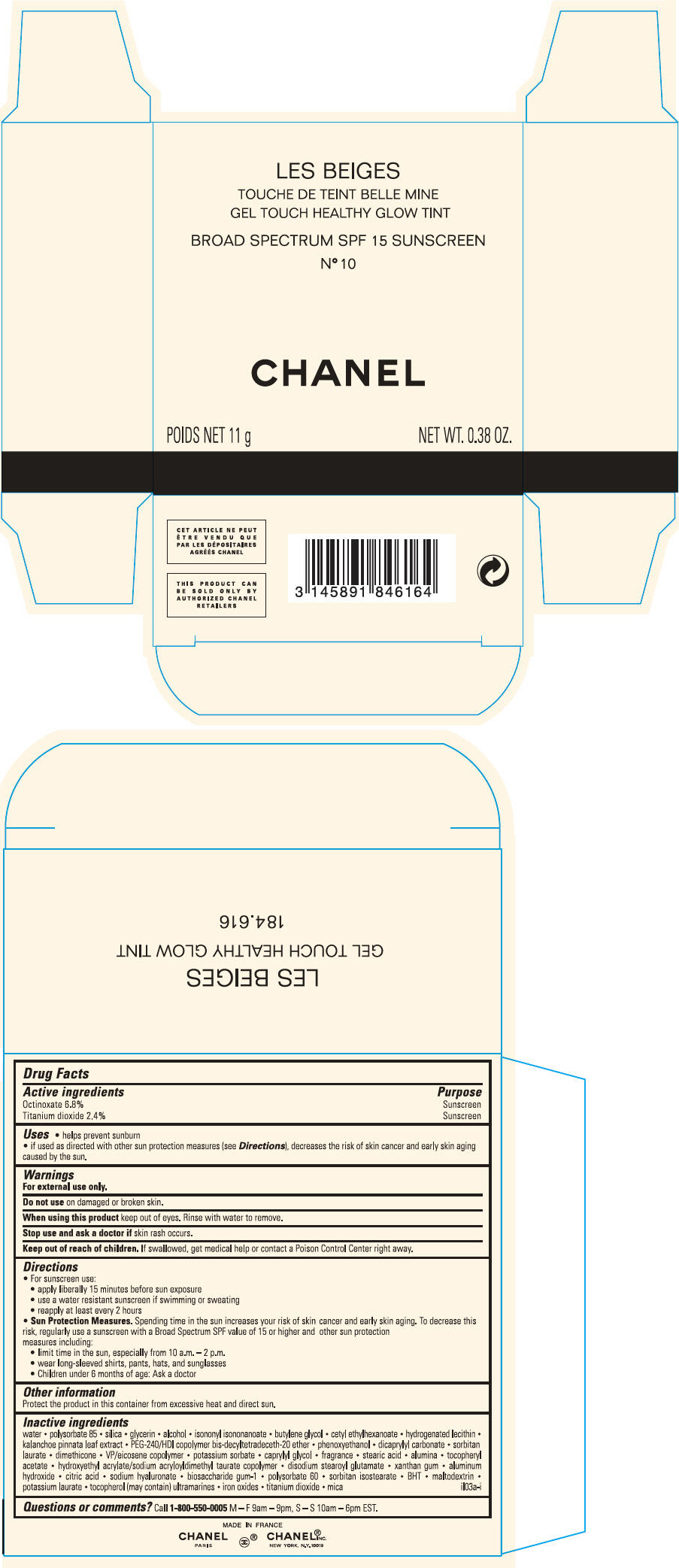
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 10
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 10
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
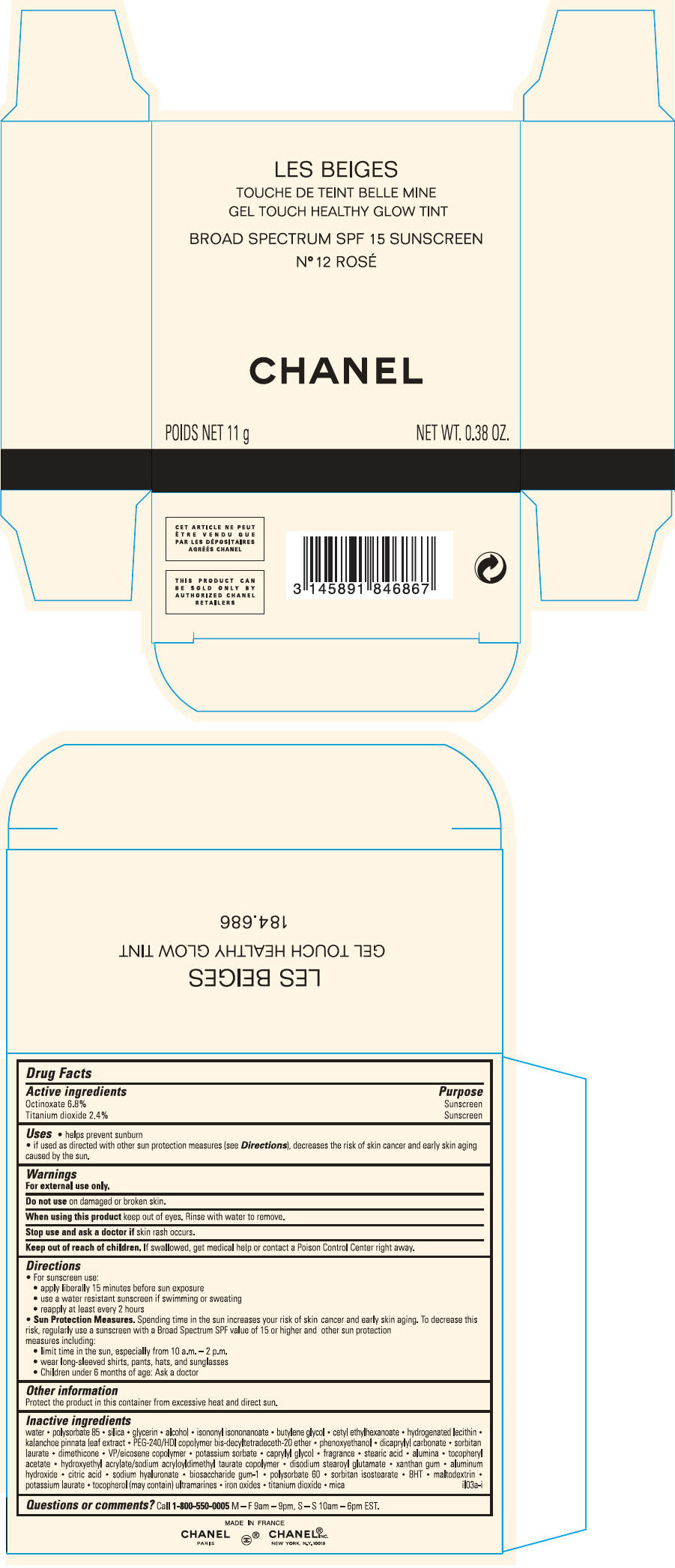
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 12 ROSÉ
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 12 ROSÉ
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 20
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 20
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
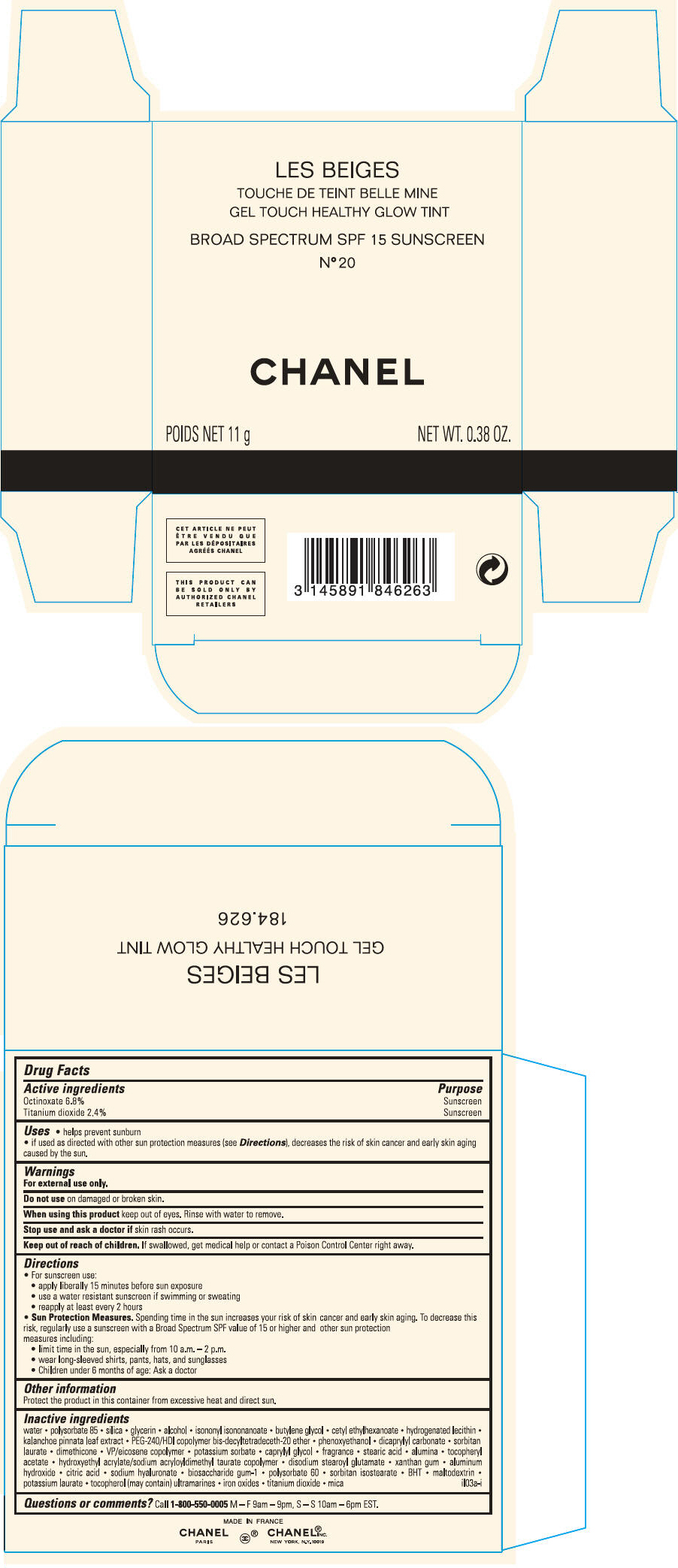
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 21
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 21
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
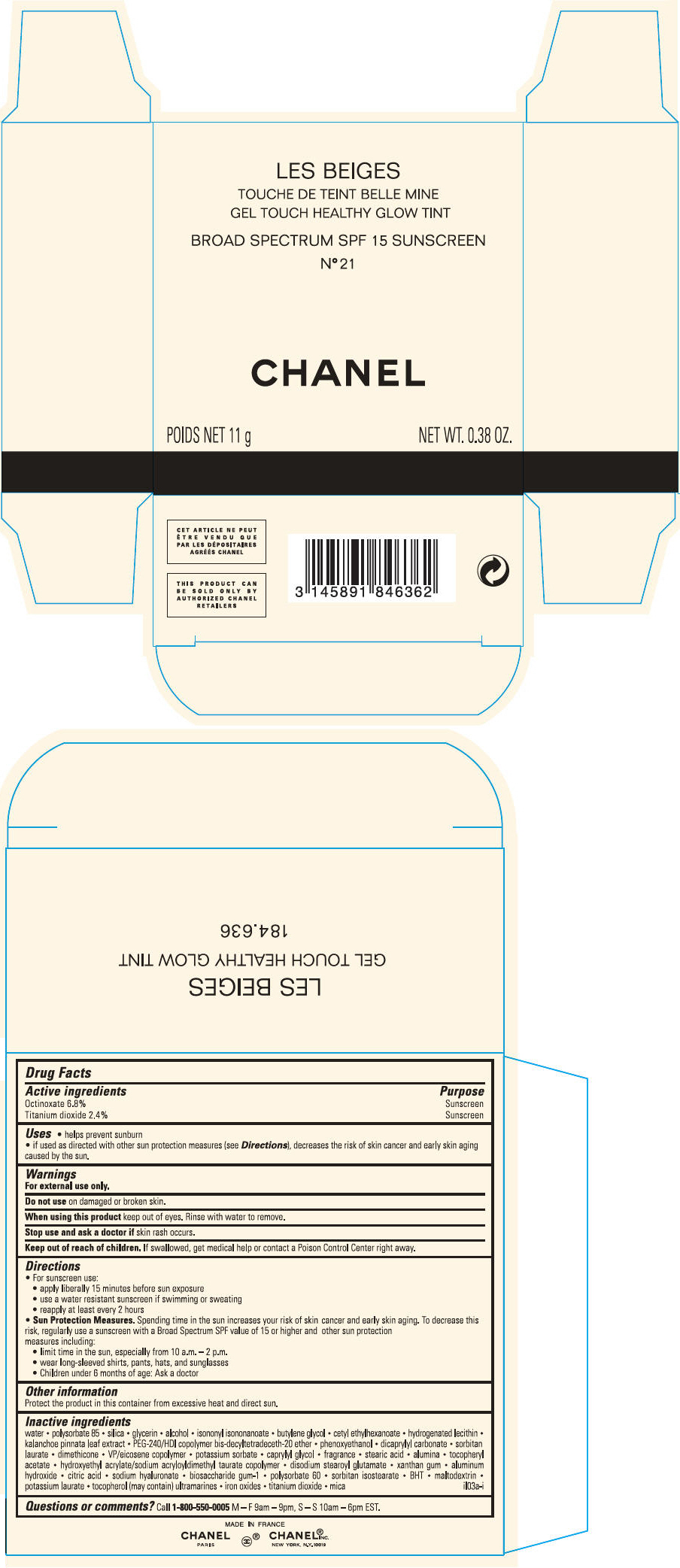
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 22 ROSÉ
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 22 ROSÉ
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
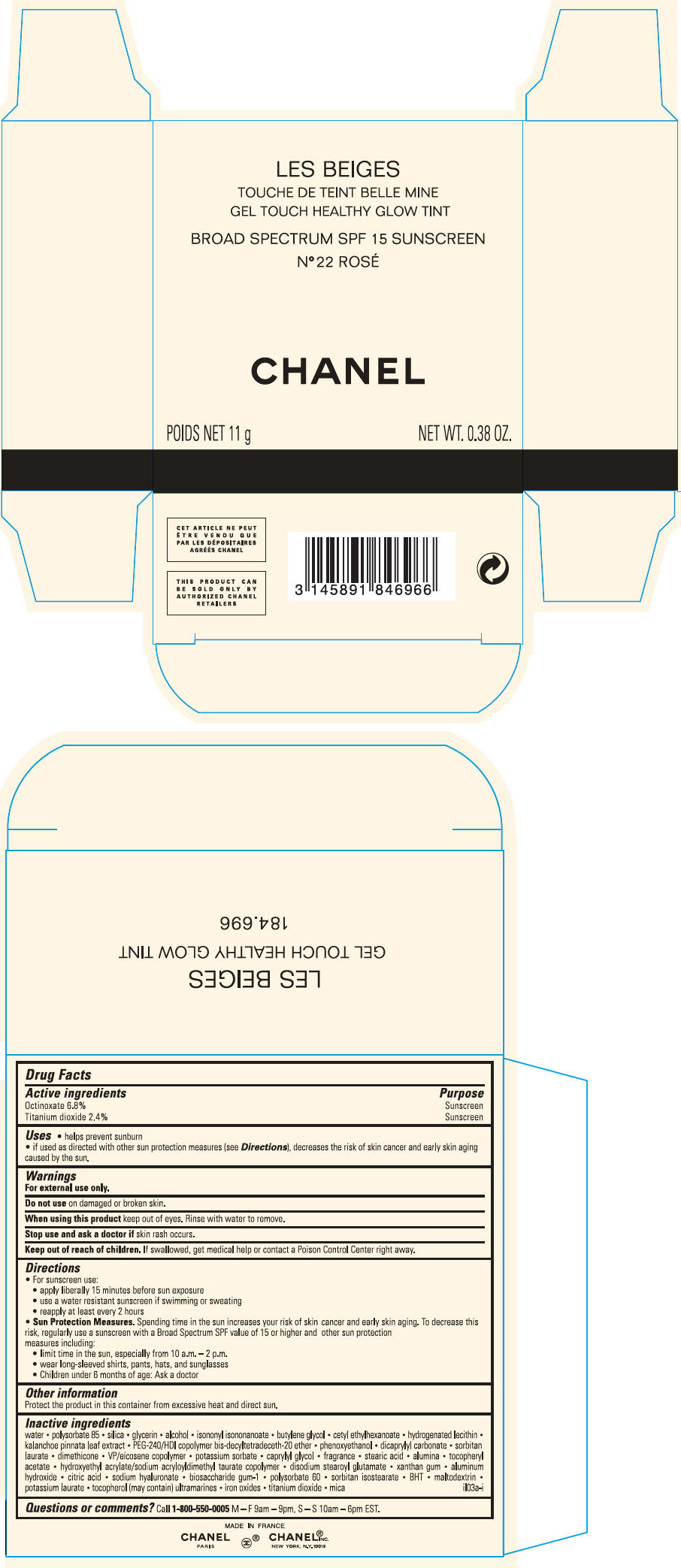
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 30
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 30
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
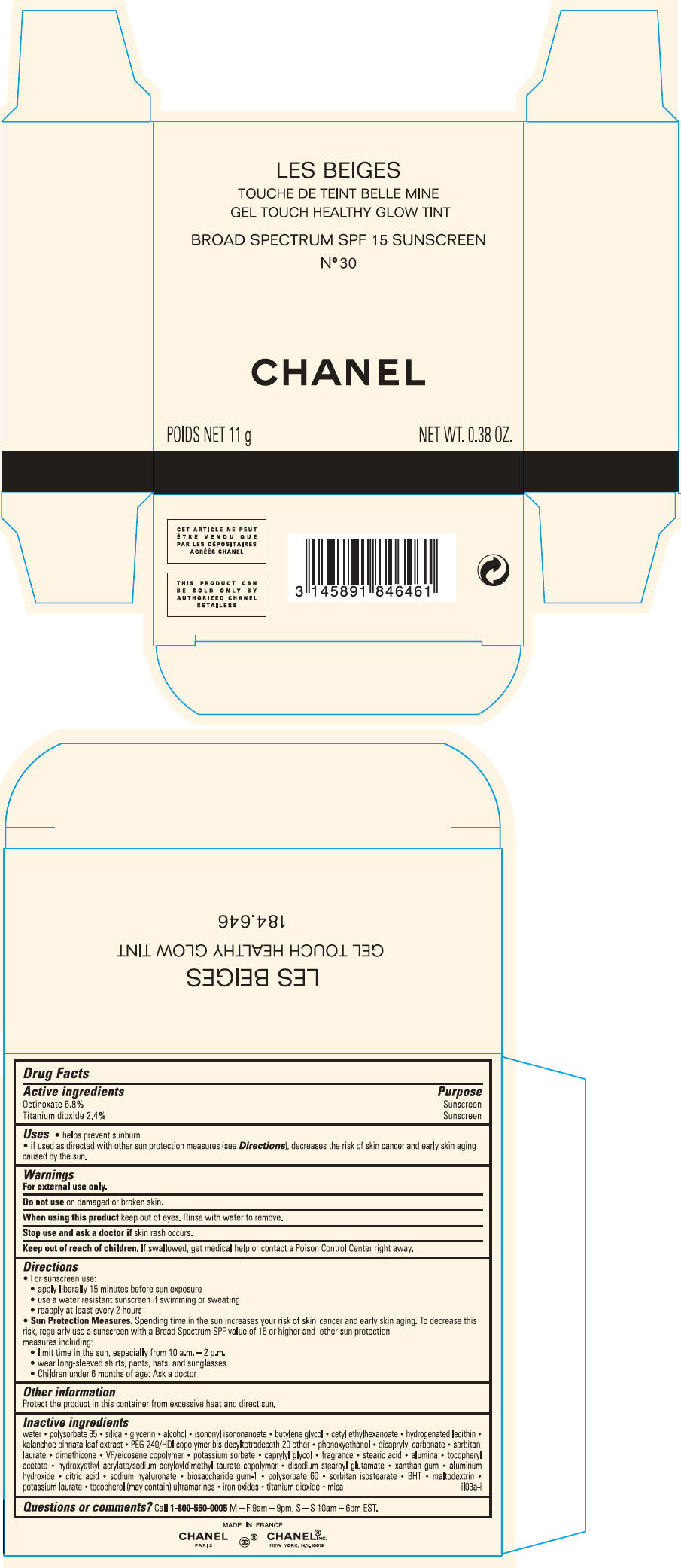
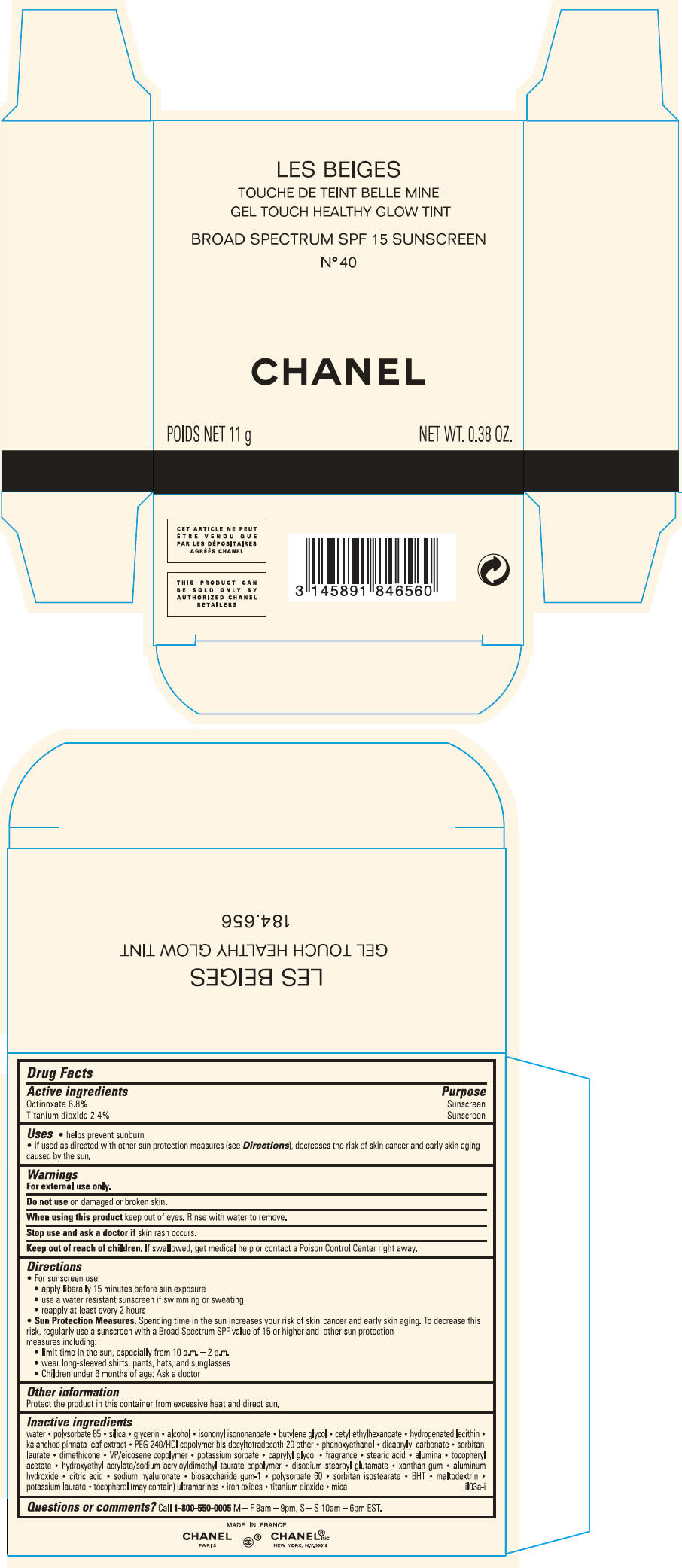
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 40
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 40
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
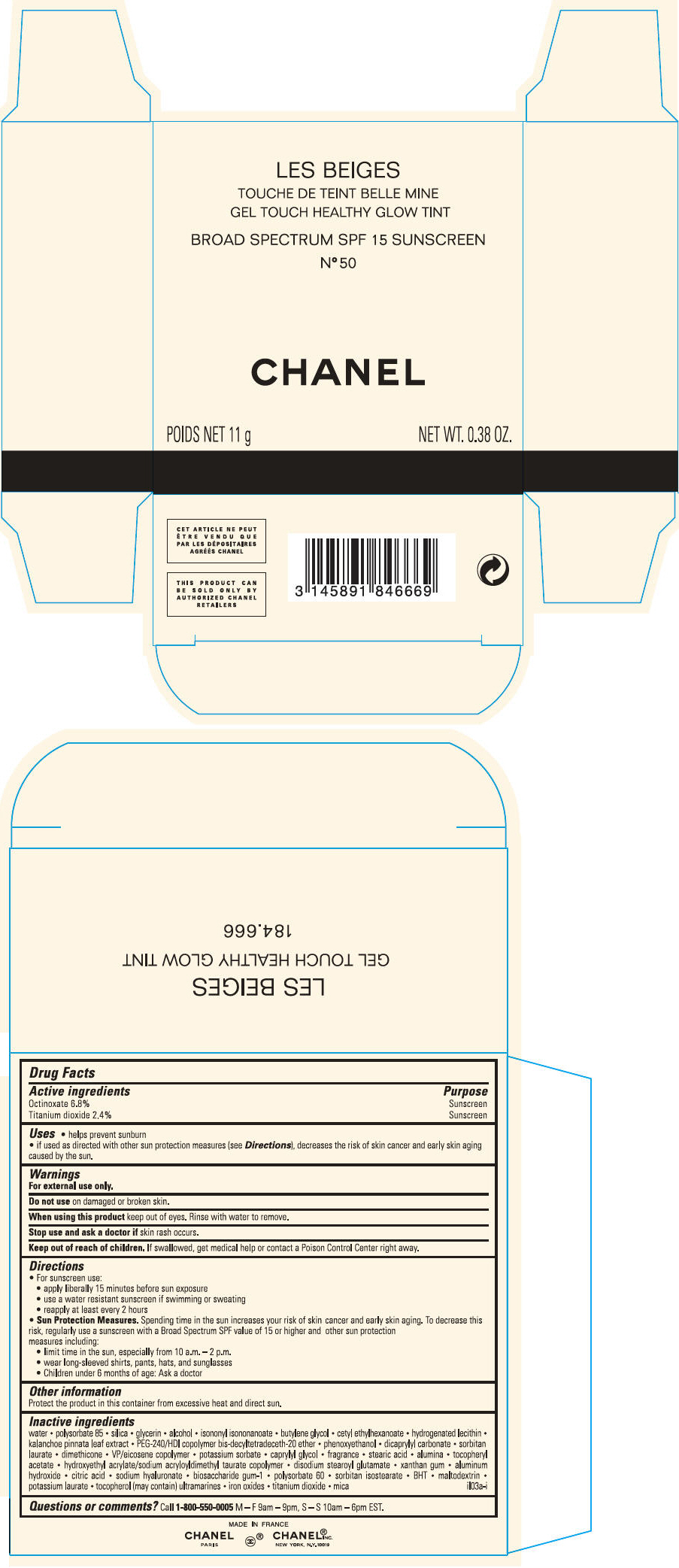
PRINCIPAL DISPLAY PANEL - 11 g Case Carton - N° 50
LES BEIGES
GEL TOUCH HEALTHY GLOW TINT
BROAD SPECTRUM SPF 15 SUNSCREEN
N° 50
CHANEL
POIDS NET 11 g
NET WT. 0.38 OZ.
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 10
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 20
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 21
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 30
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 40
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 50
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 60
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 12 ROSE
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 22 ROSE
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LES BEIGES
GEL TOUCH HEALTHY GLOW TINT BROAD SPECTRUM SPF 15 SUNSCREEN NO 91 CARAMEL
octinoxate and titanium dioxide gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CHANEL PARFUMS BEAUTE (275137669) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CHANEL PARFUMS BEAUTE | 277032509 | MANUFACTURE(68745-2080, 68745-2081, 68745-2082, 68745-2083, 68745-2084, 68745-2085, 68745-2086, 68745-2087, 68745-2088, 68745-2089) , LABEL(68745-2080, 68745-2081, 68745-2082, 68745-2083, 68745-2084, 68745-2085, 68745-2086, 68745-2087, 68745-2088, 68745-2089) | |