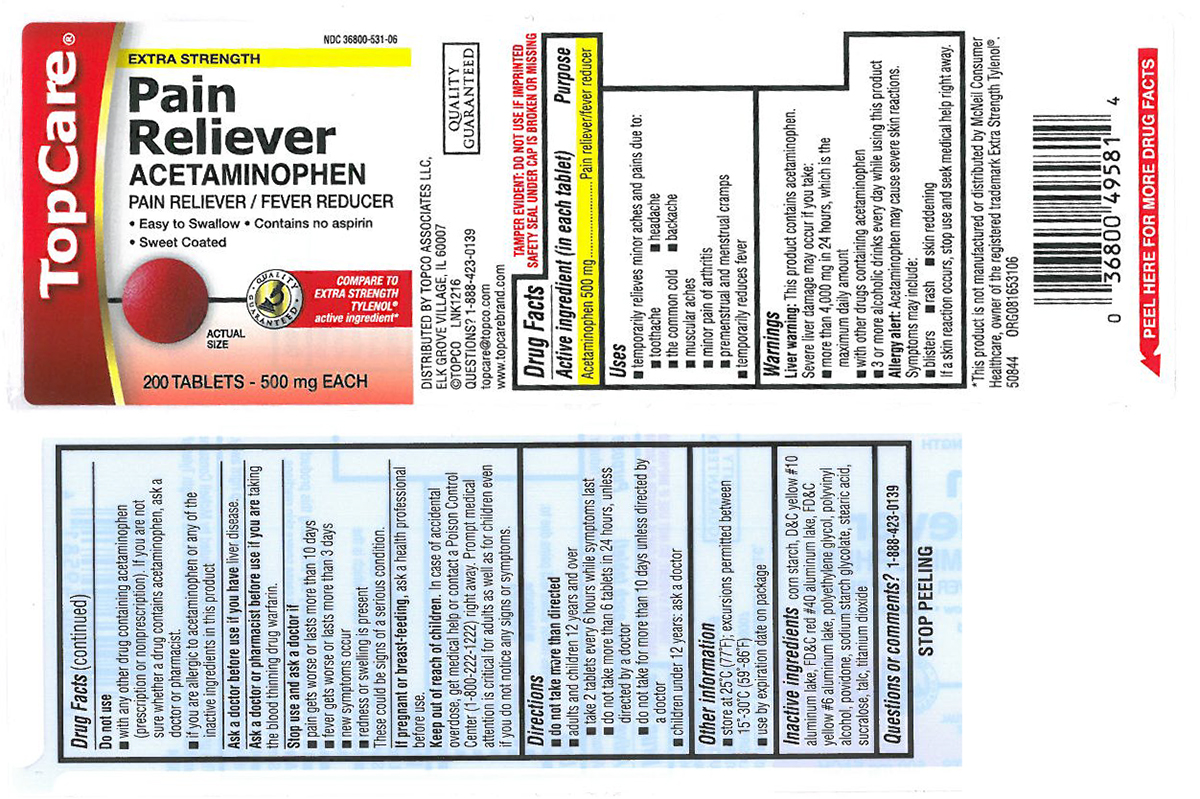
PAIN RELIEVER EXTRA STRENGTH- acetaminophen tablet, coated
Topco Associates, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TopCare 44-531-Reliever
Uses
- temporarily relieves minor aches and pains due to:
- backache
- muscular aches
- headache
- the common cold
- toothache
- minor pain of arthritis
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
-
do not take more than directed
- adults and children 12 years and over
- take 2 tablets every 6 hours while symptoms last
- do not take more than 6 tablets in 24 hours, unless directed by a doctor
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: ask a doctor
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- use by expiration date on package
Inactive ingredients
corn starch, D&C yellow #10 aluminum lake, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, polyethylene glycol, polyvinyl alcohol, povidone, sodium starch glycolate, stearic acid, sucralose, talc, titanium dioxide
Principal Display Panel
TopCare®
NDC 36800-531-06
EXTRA STRENGTH
Pain
Reliever
ACETAMINOPHEN
PAIN RELIEVER / FEVER REDUCER
• Easy to swallow
• Contains no aspirin
• Sweet coated
ACTUAL
SIZE
200 TABLETS - 500 mg EACH
COMPARE TO
EXTRA STRENGTH
TYLENOL®
active ingredient*
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
QUALITY GUARANTEED
DISTRIBUTED BY TOPCO ASSOCIATES LLC,
ELK GROVE VILLIAGE, IL 60007
©TOPCO LNK1216
QUESTIONS? 1-888-423-0139
topcare@topco.com
www.topcarebrand.com
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol®.
50844 ORG081653106
TopCare 44-531
| PAIN RELIEVER
EXTRA STRENGTH
acetaminophen tablet, coated |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Topco Associates, LLC (006935977) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | MANUFACTURE(36800-531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | PACK(36800-531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | PACK(36800-531) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 868734088 | PACK(36800-531) | |