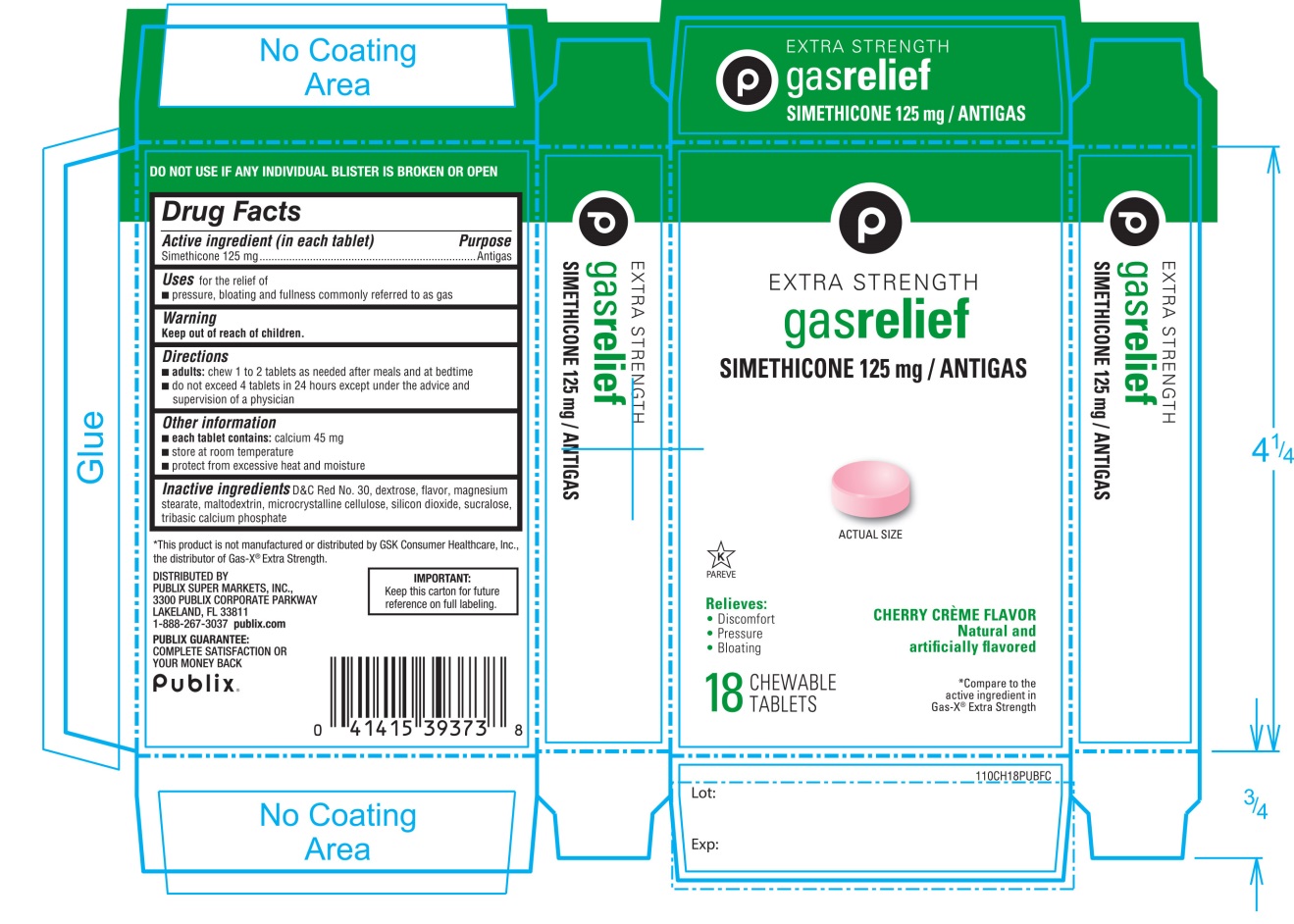
Label: EXTRA STRENGTH GAS RELIEF- simethicone tablet, chewable
- NDC Code(s): 41415-110-18
- Packager: PUBLIX SUPER MARKETS, INC,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses for relief of
- Warning
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
Publix
EXTRA STRENGTH
gas relief
SIMETHICONE 125 mg/ANTIGAS
CHERRY CREME FLAVOR
Natural and Artificially Flavored
- Relieves:
- •
- Discomfort
- •
- Pressure
- •
- Bloating
- *Compare to the active ingredient in Gas-X® Extra Strength
18 CHEWABLE TABLETS
DO NOT USE IF ANY INDIVIDUAL BLISTER IS BROKEN OR OPEN
*This product is not manufactured or distributed by GSK Consumer Healthcare Inc., the distributor of GAS-X® Extra Strength.
DISTRIBUTED BY
3300 PUBLIX CORPORATE PARKWAY
PUBLIX SUPER MARKETS, INC,
LAKELAND,FL33811
Publix.com
PUBLIX GUARANTEE:
COMPLETE SATISFACTION OR YOUR MONEY BACK
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH GAS RELIEF
simethicone tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41415-110 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) Product Characteristics Color PINK Score no score Shape ROUND Size 14mm Flavor CHERRY (Cherry Creme) Imprint Code RP110 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41415-110-18 3 in 1 CARTON 11/14/2017 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 11/14/2017 Labeler - PUBLIX SUPER MARKETS, INC, (006922009)