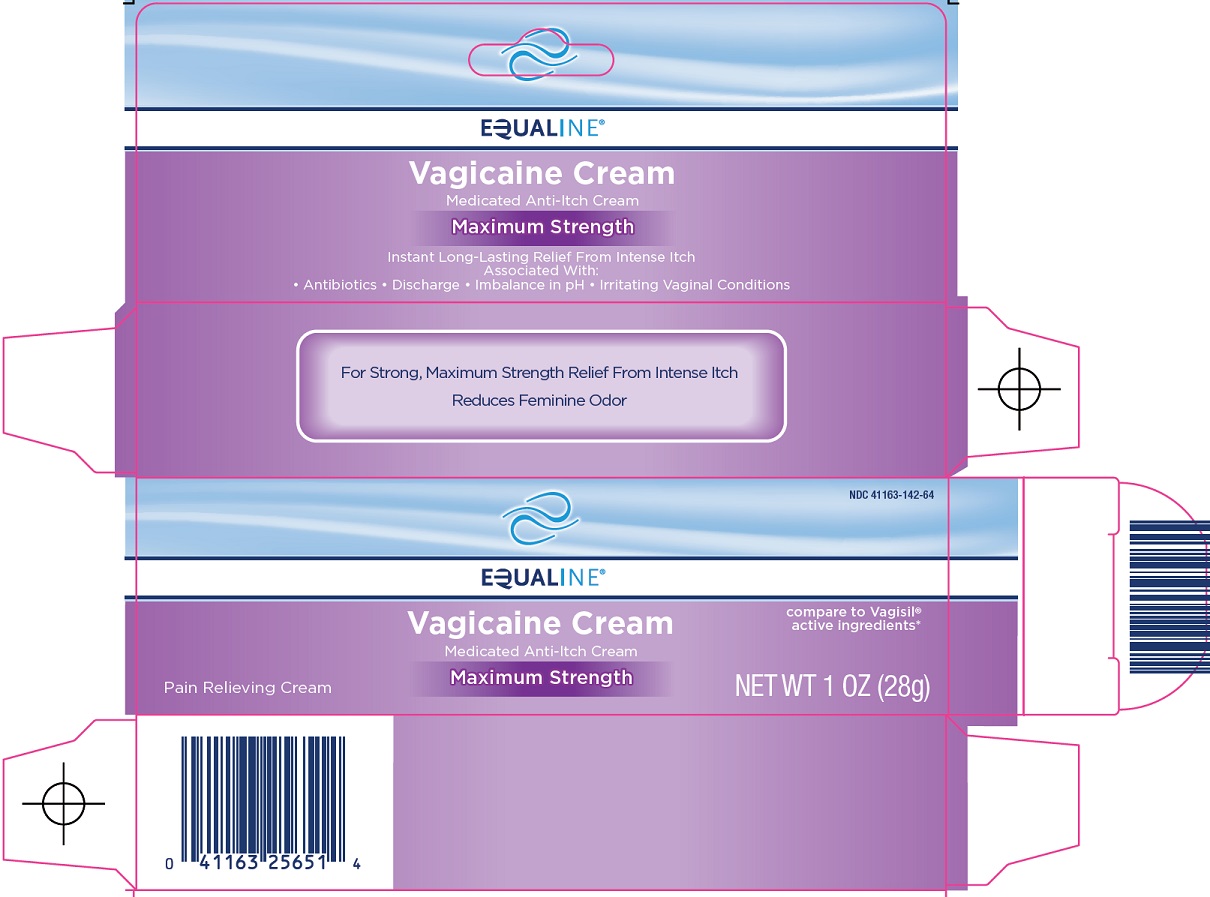
EQUALINE VAGICAINE MAXIMUM STRENGTH- benzocaine, resorcinol cream
Supervalu Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
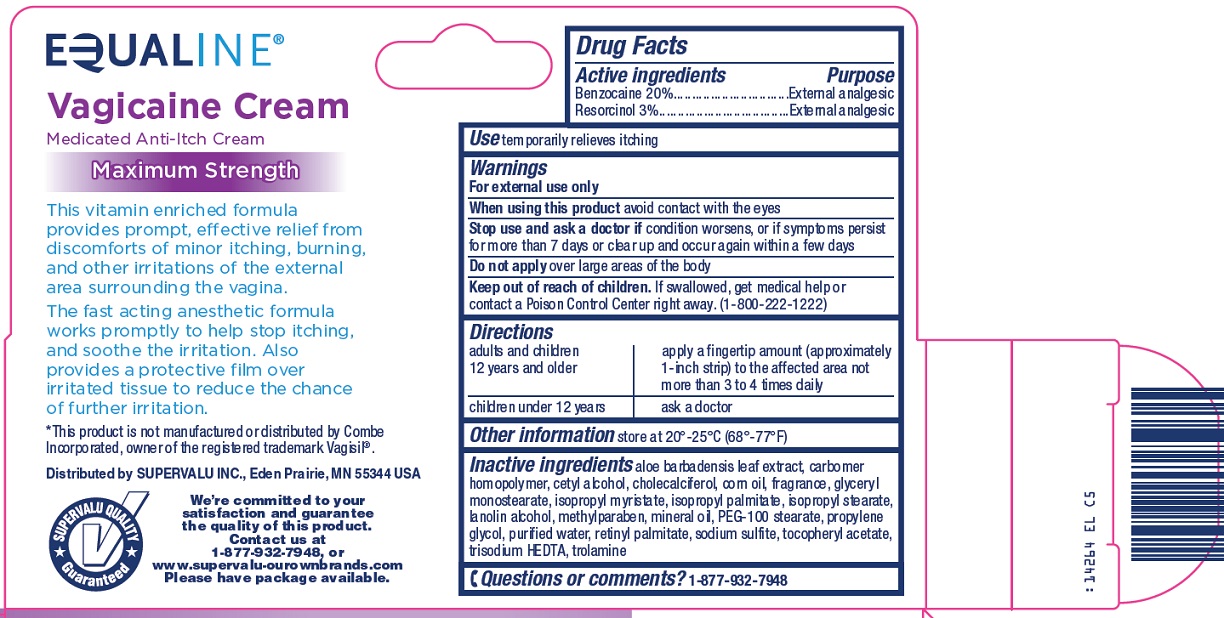
SuperValu Inc. Vagicaine Cream Drug Facts
Warnings
For external use only
Directions
|
adults and children 12 years and older |
apply a fingertip amount (approximately 1-inch strip) to the affected area not more than 3 to 4 times daily |
|
children under 12 years |
ask a doctor |
Inactive ingredients
aloe barbadensis leaf extract, carbomer homopolymer , cetyl alcohol, cholecalciferol, corn oil, fragrance, glyceryl monostearate, isopropyl myristate, isopropyl palmitate, isopropyl stearate, lanolin alcohol, methylparaben, mineral oil, PEG-100 stearate, propylene glycol, purified water, retinyl palmitate, sodium sulfite, tocopheryl acetate, trisodium HEDTA, trolamine
| EQUALINE VAGICAINE
MAXIMUM STRENGTH
benzocaine, resorcinol cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Supervalu Inc (006961411) |