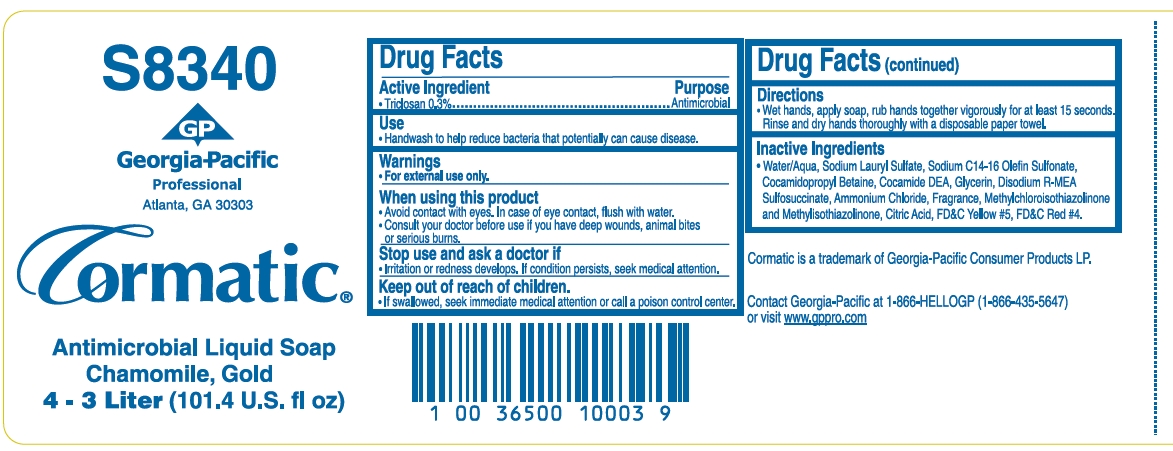
ANTIMICROBIAL- triclosan soap
Chester Packaging, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Warnings
- For external use only.
When using this product
- Avoid contact with eyes. In case of eye contact, flush with water.
- Consult your doctor before use if you have any deep wounds, animal bites or serious burns.
Directions
- Wet hands, apply soap, rub hands together vigorously for at least 15 seconds. Rinse and dry hands thoroughly with a disposable paper towel.
| ANTIMICROBIAL
triclosan soap |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Chester Packaging, LLC (004237806) |
Revised: 1/2016
Document Id: 2a3ee26f-7f8b-39e9-e054-00144ff8d46c
Set id: 0256b301-95c6-4952-8c71-32f8a16d3a01
Version: 2
Effective Time: 20160126
Chester Packaging, LLC