METRONIDAZOLE- metronidazole gel
Medicis Pharmaceutical Corp
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METRONIDAZOLE VAGINAL GEL 1.3% safely and effectively. See full prescribing information for METRONIDAZOLE VAGINAL GEL 1.3%.
METRONIDAZOLE vaginal gel 1.3% Initial U.S. Approval: 1963 INDICATIONS AND USAGEMetronidazole vaginal gel 1.3% is a nitroimidazole antimicrobial indicated for the treatment of bacterial vaginosis in non-pregnant women. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSVaginal gel 65 mg of metronidazole in 5 grams of gel (1.3%) in a prefilled applicator (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions observed in clinical studies (incidence ≥1%) were vulvovaginal candidiasis, headache, vulvovaginal pruritus, nausea, diarrhea, and dysmenorrhea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSUSE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 7/2014 |
FULL PRESCRIBING INFORMATION
1 INDICATION AND USAGE
Metronidazole vaginal gel 1.3% is indicated in the treatment of bacterial vaginosis in non-pregnant women.
2 DOSAGE AND ADMINISTRATION
A single-dose, pre-filled disposable applicator (which delivers approximately 5 g of gel containing 65 mg of metronidazole) administered once intravaginally. Metronidazole vaginal gel 1.3% should be administered at bedtime.
Metronidazole vaginal gel 1.3% is not for ophthalmic, dermal or oral use.
3 DOSAGE FORMS AND STRENGTHS
Vaginal gel (1.3%) containing 65 mg of metronidazole in 5 grams of gel in a pre-filled applicator.
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Metronidazole vaginal gel 1.3% is contraindicated in persons who have shown hypersensitivity to metronidazole, parabens, other ingredients of the formulation, or other nitroimidazole derivatives.
5 WARNINGS AND PRECAUTIONS
5.1 Central and Peripheral Nervous System Effects
Convulsive seizures and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity, have been reported in patients treated with oral or intravenous metronidazole. Metronidazole vaginal gel 1.3% should be administered with caution to patients with central nervous system diseases.
5.2Carcinogenicity in Animals
Metronidazole has been shown to be carcinogenic at high doses administered orally in mice and rats [see Nonclinical Toxicology (13.1)]. Unnecessary use of metronidazole should be avoided. Use of Metronidazole vaginal gel 1.3% should be reserved for the treatment of bacterial vaginosis [see Indications and Usage (1)].
5.3Drug/Laboratory Test Interactions
Metronidazole may interfere with certain types of determinations of serum chemistry values, such as aspartate aminotransferase (AST, SGOT), alanine aminotransferase (ALT, SGPT), lactate dehydrogenase (LDH), triglycerides, and glucose hexokinase. Values of zero may be observed. All of the assays in which interference has been reported involve enzymatic coupling of the assay to oxidation reduction of nicotinamide-adenine dinucleotides (NAD + NADH). Interference is due to the similarity in absorbance peaks of NADH (340 nm) and metronidazole (322 nm) at pH 7.
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1Clinical Trials Experience
The safety of Metronidazole vaginal gel 1.3% was evaluated in a randomized, double-blind, vehicle-controlled study in subjects with bacterial vaginosis. A total of 321 non-pregnant females with a mean age of 33.4 years (range 18 to 67 years) received Metronidazole vaginal gel 1.3%. Subjects were primarily Black/African American (58.3%) or White (39.3%). Subjects administered a single dose of Metronidazole vaginal gel 1.3% at bedtime on the first day of the study.
There were no deaths or serious adverse events in this trial. Adverse events were reported by 19.0% of subjects treated with Metronidazole vaginal gel 1.3% versus 16.1% of subjects treated with Vehicle Gel.
Adverse events occurring in ≥1% of subjects receiving Metronidazole vaginal gel 1.3% were: vulvovaginal candidiasis (5.6%), headache (2.2%), vulvovaginal pruritus (1.6%), nausea (1.6%), diarrhea (1.2%), and dysmenorrhea (1.2%). No subjects discontinued treatment due to adverse events.
6.2Other Metronidazole Formulations
Other Vaginal Formulations
Other reactions that have been reported in association with the use of other formulations of metronidazole vaginal gel include: unusual taste and decreased appetite.
Topical (Dermal) Formulations
Other reactions that have been reported in association with the use of topical (dermal) formulations of metronidazole include skin irritation, transient skin erythema, and mild skin dryness and burning. None of these adverse reactions exceeded an incidence of 2% of patients.
Oral and Parenteral Formulations
The following adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of metronidazole:
Cardiovascular: Flattening of the T-wave may be seen in electrocardiographic tracings.
Nervous System: The most serious adverse reactions reported in patients treated with oral metronidazole have been convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity. In addition, patients have reported syncope, vertigo, incoordination, ataxia, confusion, dysarthria, irritability, depression, weakness, and insomnia [see Warnings and Precautions (5.1)].
Gastrointestinal: Abdominal discomfort, nausea, vomiting, diarrhea, an unpleasant metallic taste, anorexia, epigastric distress, abdominal cramping, constipation, “furry” tongue, glossitis, stomatitis, pancreatitis, and modification of taste of alcoholic beverages.
Genitourinary: Overgrowth of Candida in the vagina, dyspareunia, decreased libido, proctitis.
Hematopoietic: Reversible neutropenia, reversible thrombocytopenia.
Hypersensitivity Reactions: Urticaria; erythematous rash; Stevens-Johnson Syndrome, toxic epidermal necrolysis, flushing; nasal congestion; dryness of the mouth, vagina, or vulva; fever; pruritus; fleeting joint pains [see Contraindications (4)].
Renal: Dysuria, cystitis, polyuria, incontinence, a sense of pelvic pressure, darkened urine.
7 DRUG INTERACTIONS
The intravaginal administration of a single dose of Metronidazole vaginal gel 1.3% results in lower systemic exposure to metronidazole that is approximately 2% to 4% of that achieved following oral administration of 500 mg metronidazole tablets [see Clinical Pharmacology (12.3)]. The following drug interactions were reported for oral metronidazole.
7.1 Disulfiram
Use of oral metronidazole has been associated with psychotic reactions in alcoholic patients who are using disulfiram concurrently. Metronidazole vaginal gel 1.3% should not be used by patients who have taken disulfiram within the last two weeks [see Contraindications (4.2)].
7.2 Alcoholic Beverages
Use of oral metronidazole has been associated with a disulfiram-like reaction (abdominal cramps, nausea, vomiting, headaches, and flushing) to alcohol. Alcoholic beverages and preparations containing ethanol or propylene glycol should not be consumed during and for at least 24 hours after Metronidazole vaginal gel 1.3% therapy [see Contraindications (4.3)].
7.3 Coumarin and Other Oral Anticoagulants
Use of oral metronidazole has been reported to potentiate the anticoagulant effect of warfarin and other coumarin anticoagulants, resulting in a prolongation of prothrombin time. This possible drug interaction should be considered when Metronidazole vaginal gel 1.3% is prescribed for patients on this type of anticoagulant therapy.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
Metronidazole vaginal gel 1.3% should be used during pregnancy only if clearly needed. There are no adequate and well-controlled studies in pregnant women. Metronidazole crosses the placental barrier and enters the fetal circulation rapidly.
No fetotoxicity or teratogenicity was observed when metronidazole was administered orally to pregnant rabbits at up to 200 mg/kg (about 60 times the maximum human dose based on mg/m2). Similarly no fetotoxic or teratogenic effects were observed in five studies in rats where dosing was administered orally in the diet or by gastric intubation at doses up to 200 mg/kg (about 30 times the maximum human dose based on mg/m2).
As well, no fetotoxicity or teratogenicity was observed when metronidazole was administered orally to pregnant mice at doses up to 100 mg/kg (about 7 times the maximum human dose based on mg/m2). However, some intrauterine deaths were observed in Swiss Webster mice administered metronidazole intraperitoneally at doses up to 15 mg/kg (about 1 times the maximum human dose based on mg/m2). The relationship of these intraperitoneal findings in mice to the topical use of Metronidazole vaginal gel 1.3% is unknown.
Because animal reproduction and development studies are not always predictive of human response, and because metronidazole is a carcinogen in rodents, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Following oral metronidazole administration, concentrations of metronidazole in human milk are similar to concentrations in plasma. Since some metronidazole is systemically absorbed following vaginal administration of Metronidazole vaginal gel 1.3%, excretion in human milk following topical use is possible.
Because of the potential for tumorigenicity shown for metronidazole in animal studies, a decision should be made whether to discontinue nursing or to discontinue Metronidazole vaginal gel 1.3%, taking into account the importance of the therapy to the mother. A nursing mother may choose to pump and discard her milk during Metronidazole vaginal gel 1.3% therapy and 24 hours after therapy ends and feed her infant stored human milk or formula.
10 OVERDOSAGE
There is no human experience with overdosage of metronidazole vaginal gel. Vaginally applied Metronidazole vaginal gel 1.3% could be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5) and Adverse Reactions (6.2)].
11 DESCRIPTION
METRONIDAZOLE vaginal gel, 1.3% contains 1.3% metronidazole, USP in a single-dose prefilled disposable applicator. It is intended for intravaginal use. Metronidazole is a nitroimidazole antimicrobial.
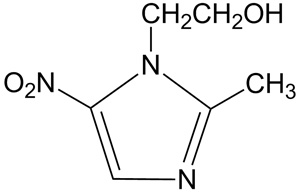
Chemically, metronidazole is a 2-methyl-5-nitroimidazole-1-ethanol. Its structural formula is:
The molecular formula is C6H9N3O3 with a molecular weight of 171.16.
Metronidazole vaginal gel 1.3% is an aqueous gel containing metronidazole at a concentration of 13 mg/g (1.3%). The gel is formulated at pH 4.0. The gel also contains benzyl alcohol, methylparaben, polycarbophil, polyethylene glycol 400, propylene glycol, propylparaben, and purified water..
Each applicator contains approximately 65 mg of metronidazole in 5 g of vaginal gel.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metronidazole is an antimicrobial drug [see Clinical Pharmacology, Microbiology (12.4)].
12.3 Pharmacokinetics
Following a single, intravaginal 5 g dose of Metronidazole vaginal gel 1.3% (equivalent to 65 mg of metronidazole) to 20 healthy female subjects, a mean maximum serum metronidazole concentration (Cmax) of 239 ng/mL was observed (range: 114 to 428 ng/mL). The average time to achieve this Cmax was 7.3 hours (range: 4 to 18 hours). This Cmax is approximately 2% of the mean maximum serum concentration reported in healthy subjects administered a single, oral 500 mg dose of metronidazole tablets (mean Cmax = 12,785 ng/mL).
The extent of exposure [area under the curve (AUC)] of metronidazole, when administered as a single intravaginal 5 g dose of Metronidazole vaginal gel 1.3% (equivalent to 65 mg of metronidazole), was 5,434 ng•hr/mL (range: 1382 to 12744 ng•hr/mL). This AUC0-∞ is approximately 4% of the reported AUC of metronidazole following a single oral 500 mg dose of metronidazole (approximately 125,000 ng•hr/mL).
12.4 Microbiology
Metronidazole, is a nitroimidazole antimicrobial agent that acts primarily against anaerobic bacteria and selected protozoa. The 5-nitro group on the metronidazole molecule is reduced by metabolically active anaerobes to its active state by the bacterial nitro-reductase enzyme after it diffuses into the bacterial cell. This results in the production of cytotoxic compounds that disrupt the helical structure of bacterial DNA thereby inhibiting bacterial nucleic acid synthesis which leads to cell death.
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis [see Clinical Studies (14)].
Metronidazole is active in vitro against most isolates of the following organisms that have been reported to be associated with bacterial vaginosis:
Bacteroides spp.
Gardnerella vaginalis
Mobiluncus spp.
Peptostreptococcus spp.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Metronidazole has shown evidence of carcinogenic activity in a number of studies involving chronic oral administration in mice and rats. Pulmonary tumors were reported in several mouse studies in which mice were dosed orally at 75 mg/kg and above (about 6 or more times the maximum recommended human dose based on mg/m2). Malignant lymphoma was reported at 66 mg/kg and above (about 5 or more times the maximum recommended human dose based on mg/m2). These tumors have been observed in all six reported studies in the mouse, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only). All these effects were statistically significant.
There were statistically significant increases in the incidence of mammary tumors, among female rats administered metronidazole at 270 mg/kg and above (about 40 times the maximum human dose based on mg/m2). Hepatic adenomas and carcinomas were observed in rats administered 300 mg/kg (about 45 times the maximum human dose based on mg/m2).
Two lifetime oral tumorigenicity studies in hamsters have been performed and reported to be negative at doses up to 80 mg/kg (about 10 times the maximum human dose based on mg/m2).
Carcinogenesis studies have not been conducted with Metronidazole vaginal gel 1.3%.
Although metronidazole has shown in vitro mutagenic activity in bacterial reverse mutation tests, it was negative in in vitro mammalian mutation systems including CHO/HGPRT and CH V79 lung cell assays. Metronidazole was not clastogenic in vitro chromosome aberration tests in CHO cells up to 5000 μg/mL but was positive in human and monkey peripheral blood lymphocytes at 0.1 μg/mL
In general, numerous micronucleus studies in rats and mice have failed to demonstrate a potential for genetic damage up to single oral doses 3000 mg/kg in mice (about 225 times the maximum human dose based on mg/m2). However, a dose dependent increase in the frequency of micronuclei was observed in CFW mice after intraperitoneal injections of up to 160 mg/kg (about 12 times the maximum human dose based on mg/m2).
Fertility studies have been performed in mice orally dosed up to 500 mg/kg (about 37 times the maximum human dose based on mg/m2) revealed no evidence of impaired fertility.
While no effects on fertility were observed in female rats dosed intraperitoneally at doses up to 1000 mg/kg (about 300 times the maximum human dose based on mg/m2), studies in male rats resulted in effects on testes and sperm production at oral doses of 100 mg/kg and above (about 30 times the maximum human dose based on mg/m2).
14 CLINICAL STUDIES
A single, randomized, double-blind, vehicle-controlled clinical trial was conducted to evaluate the efficacy of Metronidazole vaginal gel 1.3%. Subjects had a clinical diagnosis of bacterial vaginosis defined by the presence of a homogenous vaginal discharge that (a) had a pH ≥4.7, (b) emitted a “fishy” amine odor when mixed with a 10% KOH solution (“whiff” test), and (c) contained clue cells ≥20% of the total vaginal epithelial cells. In addition, to be eligible for analysis subjects must have had a Gram stain result ≥4 and have been negative for N. gonorrhoeae and C. trachomatis. Non-pregnant females at least 18 years of age were randomized 1:1 to either Metronidazole vaginal gel 1.3% or Vehicle Gel and instructed to administer study drug once at bedtime. Two hundred ninety-two (292) Metronidazole vaginal gel 1.3% subjects and 285 Vehicle Gel subjects were eligible for the analysis.
Clinical cure was defined as (a) return of normal physiologic discharge, (b) negative KOH “whiff” test, and (c) clue cell <20% of the total vaginal epithelial cells at the Test of Cure visit (between 21 to 30 days post-treatment). Bacteriological Cure was defined as a Nugent score of <4 and Therapeutic cure was defined as clinical cure and bacteriological cure. Metronidazole vaginal gel 1.3% demonstrated statistically significantly higher cure rates over Vehicle Gel as measured by clinical cure, bacteriological cure and therapeutic cure (Table 1).
| Outcome | Metronidazole vaginal gel 1.3%
N = 292 n (%) | Vehicle Gel
N = 285 n (%) | Treatment Difference (%)
[95% Confidence Interval] |
|---|---|---|---|
|
Test of Cure (Day 21 to 30) | |||
|
Clinical Cure |
108 (37.0) |
76 (26.7) |
10.3 (2.8, 17.9) |
|
Bacteriological Cure |
57 (19.5) |
22 (7.7) |
11.8 (6.3, 17.3) |
|
Therapeutic Cure |
49 (16.8) |
18 (6.3) |
10.5 (5.3, 15.6) |
Clinical Cure and Bacteriological Cure were also assessed at Day 7. Clinical Cure at Day 7 was achieved by a statistically significantly greater proportion of subjects in the Metronidazole Vaginal Gel 1.3% group compared to subjects in the Vehicle Gel group (41.1% vs. 20.0%). Bacteriological Cure at Day 7 was achieved by a statistically significantly greater proportion of subjects in the Metronidazole Vaginal Gel 1.3% group compared to subjects in the Vehicle Gel group (33.9% vs. 6.3%).
16 HOW SUPPLIED/STORAGE AND HANDLING
Metronidazole vaginal gel 1.3% is available in cartons containing one single-dose, prefilled disposable applicator delivering 5 g of vaginal gel containing approximately 65 mg of metronidazole:
5 g disposable applicator (NDC 99207-140-05)
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
17.1 Interaction with Alcohol
Instruct the patient not to consume alcoholic beverages and preparations containing ethanol or propylene glycol during and for at least 24 hours after treatment with Metronidazole vaginal gel 1.3% [see Contraindications (4.3) and Drug Interactions (7.2)].
17.2 Drug Interactions
Instruct the patient not to use Metronidazole vaginal gel 1.3% if disulfiram had been used within the last two weeks [see Contraindications (4.2)], and to inform their healthcare provider if they are taking oral anticoagulants, or lithium [see Drug Interactions (7.3, 7.4)].
17.3 Vaginal Intercourse and Use with Vaginal Products
Instruct the patient not to engage in vaginal intercourse, or use other vaginal products (such as tampons or douches) following the single administration of Metronidazole vaginal gel 1.3%.
17.4 Human Milk Feeding
Advise women that they may consider discontinuing milk feeding or pump and discard their milk during treatment and for 24 hours after treatment with Metronidazole vaginal gel 1.3% [see Use in Specific Populations (8.3)].
PATIENT INFORMATION
Metronidazole (metro-NI-da-zole) vaginal gel 1.3%
For intravaginal use only. Do not use in the eyes, mouth or skin.
What is Metronidazole vaginal gel 1.3%?
Metronidazole vaginal gel 1.3% is used to treat bacterial vaginosis in women who are not pregnant.
Who should not use Metronidazole vaginal gel 1.3%?
Do not use Metronidazole vaginal gel 1.3% if you:
- •
- are allergic to metronidazole, parabens, nitroimidazole derivatives, or any of the ingredients in Metronidazole vaginal gel 1.3%. See the end of this leaflet for a complete list of ingredients in Metronidazole vaginal gel 1.3%.
- •
- take or have taken a medicine called Antabuse (disulfiram) in the last 2 weeks.
- •
- drink alcohol. Do not drink alcohol while you use Metronidazole vaginal gel 1.3% and for at least 24 hours after you stop using it. It can increase your chances of getting serious side effects.
Before you use Metronidazole vaginal gel 1.3%, tell your healthcare provider about all your medical conditions, including if you:
- •
- are pregnant or plan to become pregnant. It is not known if Metronidazole vaginal gel 1.3% will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. Metronidazole vaginal gel 1.3% passes into breast milk and may harm your baby. You and your healthcare provider should decide if you will use Metronidazole vaginal gel 1.3% or breastfeed.
Tell your healthcare provider all about the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I use Metronidazole vaginal gel 1.3%?
- •
- See the “Instructions for Use” at the end of this Patient Information leaflet for detailed instructions about how to use Metronidazole vaginal gel 1.3%
- •
- Use Metronidazole vaginal gel 1.3% exactly as your healthcare provider tells you to
- •
- Metronidazole vaginal gel 1.3% is used one time at bedtime
- •
- If you get Metronidazole vaginal gel 1.3% in your eyes, rinse your eyes with cool tap water and call your healthcare provider
What should I avoid while using Metronidazole vaginal gel 1.3%?
Do not have vaginal intercourse or use other vaginal products (such as tampons or douches).
What are the possible side effects of Metronidazole vaginal gel 1.3%?
The most common side effects of Metronidazole vaginal gel 1.3% include yeast infections, headache, vaginal itching, nausea, diarrhea, and pain with menstrual cycle.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the side effects of Metronidazole vaginal gel 1.3%. For more information, ask your healthcare provider or pharmacist. Call your healthcare provider for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about Metronidazole vaginal gel 1.3%
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Metronidazole vaginal gel 1.3% for a condition for which it was not prescribed. Do not give Metronidazole vaginal gel 1.3% to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Metronidazole vaginal gel 1.3%. If you would like more information, talk with your doctor. You can also ask your pharmacists or doctor for information about Metronidazole vaginal gel 1.3% that is written for health professionals.
For more information call: Valeant Pharmaceuticals North America LLC at 1-800-321-4576
What are the ingredients in Metronidazole vaginal gel 1.3%?
Active ingredients: metronidazole
Inactive ingredients: polyethylene glycol 400, propylene glycol, benzyl alcohol, methylparaben, propylparaben, purified water, and polycarbophil AA-1
Instructions for Use
Metronidazole vaginal gel 1.3%
For Vaginal Use Only
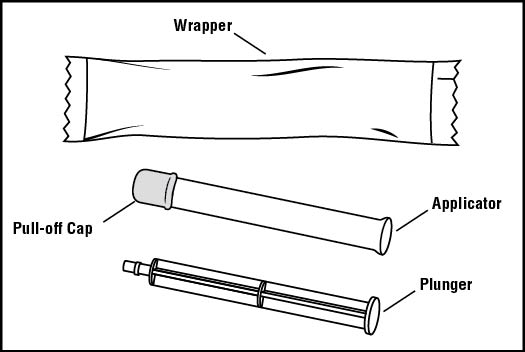
You will need the following supplies (See Figure A)
Figure A
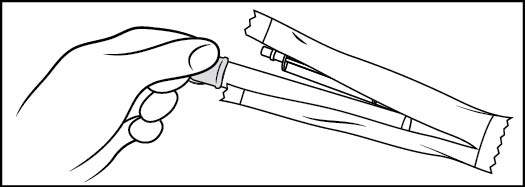
Step 1: Remove the pre-filled applicator and plunger from the foil package (See Figure B)
- •
- Tear open the foil packet just before using
- •
- Remove the pre-filled applicator and plunger from the foil packaging
Figure B
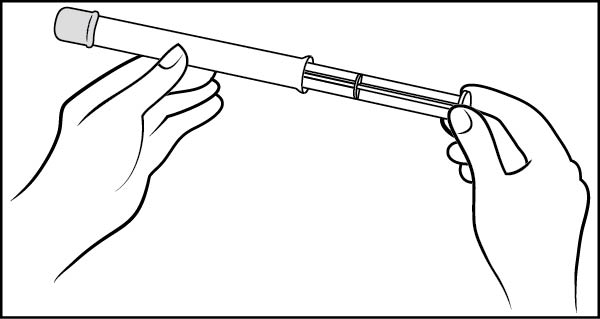
Step 2: Insert the plunger into the open end of the pre-filled applicator (See Figure C)
- •
- With the pink cap still on, push the tip of the plunger into the open end of the pre-filled applicator
Figure C
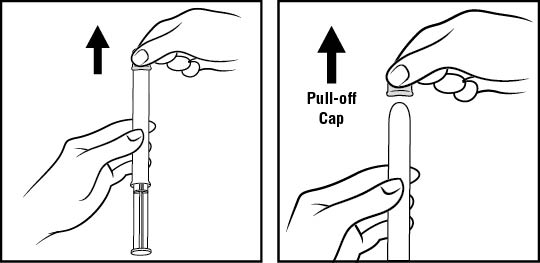
Step 3: Remove the pink cap (See Figure D)
- •
- Pull the pink cap straight off the top of the pre-filled applicator.
Figure D
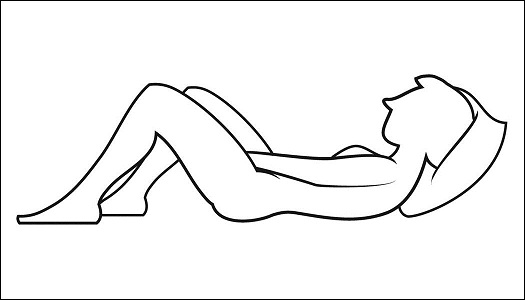
Step 4: Prepare to insert the pre-filled applicator (See Figure E)
- •
- The pre-filled applicator may be inserted while lying on your back with your knees bent or in any comfortable position.
Figure E
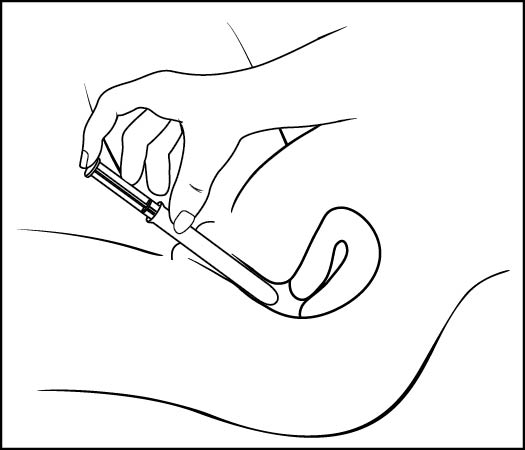
Step 5: Insert the pre-filled applicator (See Figure F)
- •
- Hold the pre-filled applicator by the barrel and gently insert the rounded tip into your vagina as far as it will comfortably go, then pull back slightly
Figure F
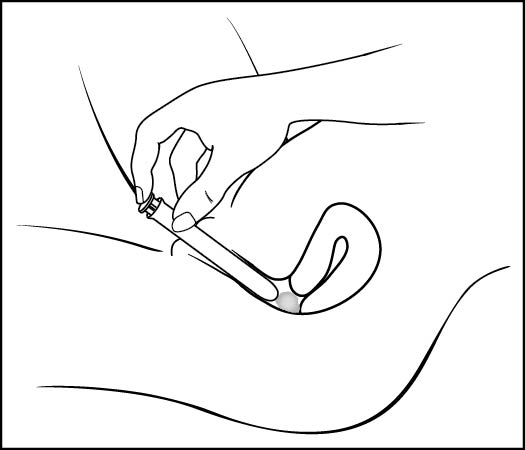
Step 6: Push the plunger (See Figure G)
- •
- While holding the barrel in place, slowly press the plunger until it stops to release the gel into your vagina
Figure G
Step 7: Remove the pre-filled applicator from your vagina and throw it away in your household trash.
How should I store Metronidazole vaginal gel 1.3%?
- •
- Store Metronidazole vaginal gel 1.3% at room temperature, 59°-86°F (15°-30°C).
Keep Metronidazole vaginal gel 1.3% and all medicines out of the reach of children.
This Patient Information and Instructions for Use have been approved by the US Food and Drug Administration.
Rx only
Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215
U.S. Patent 7,893,097
Rev. 03/2014
9386300
| METRONIDAZOLE
metronidazole gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Medicis Pharmaceutical Corp (182837492) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | MANUFACTURE(99207-140) | |