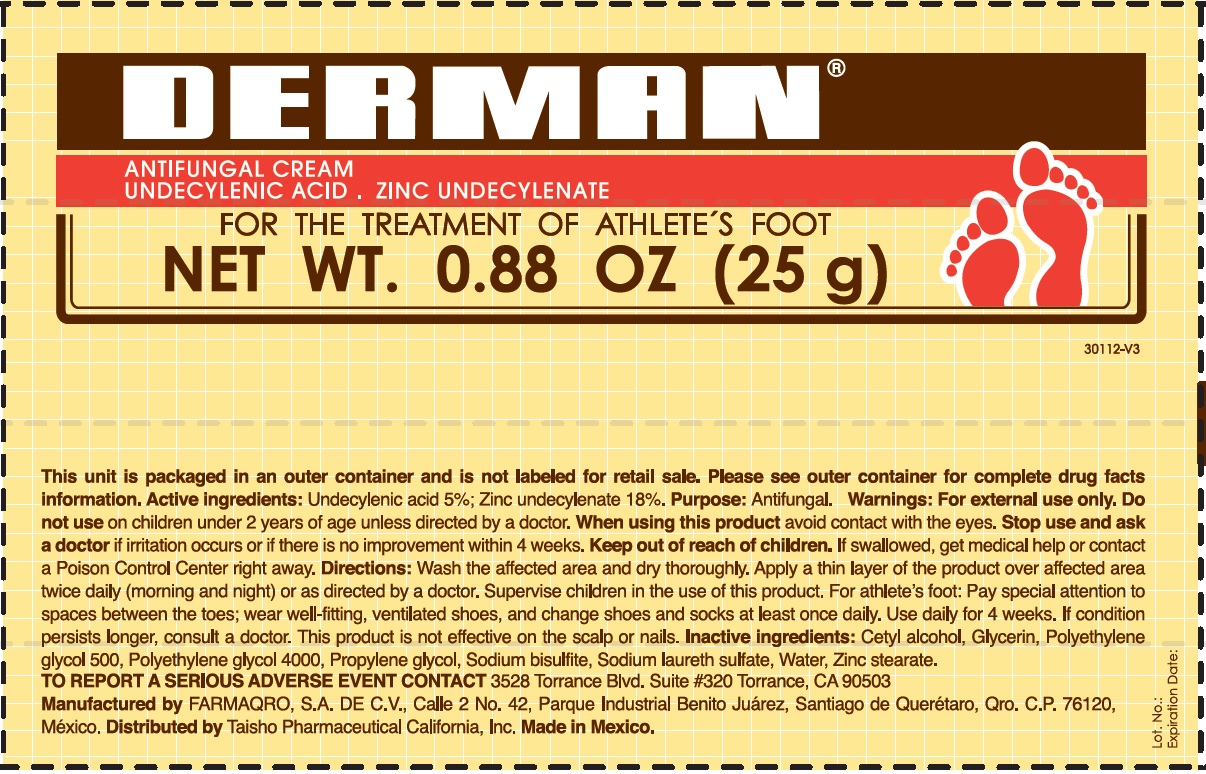
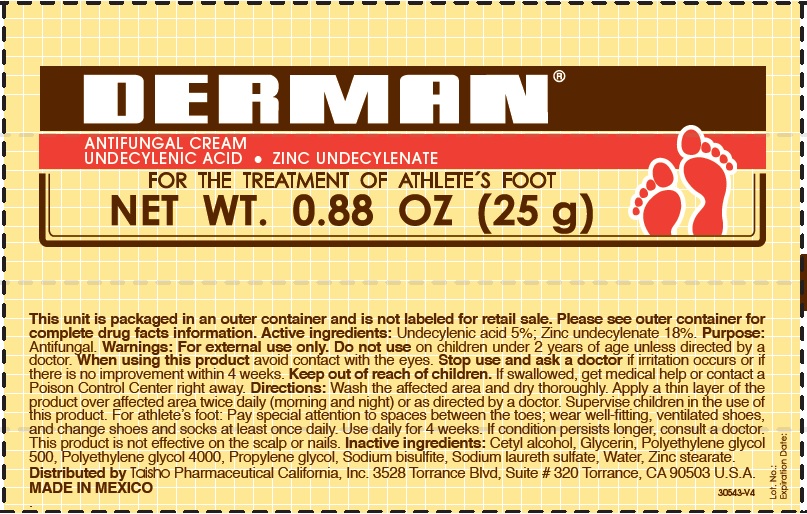
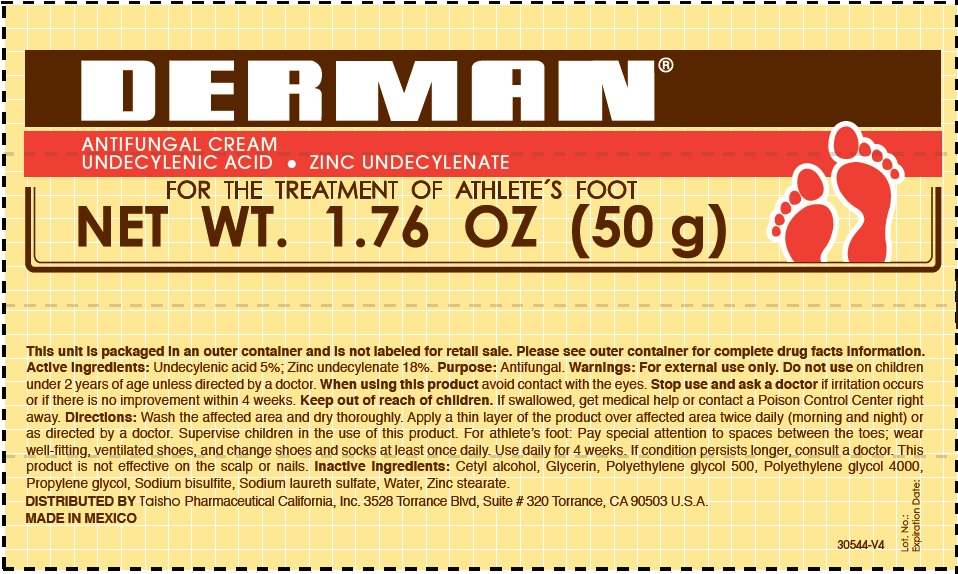
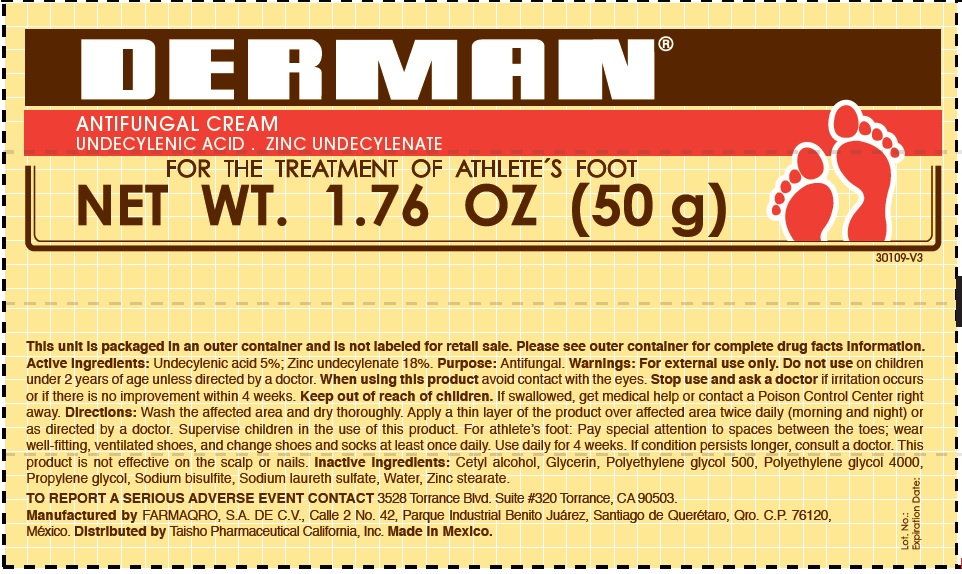
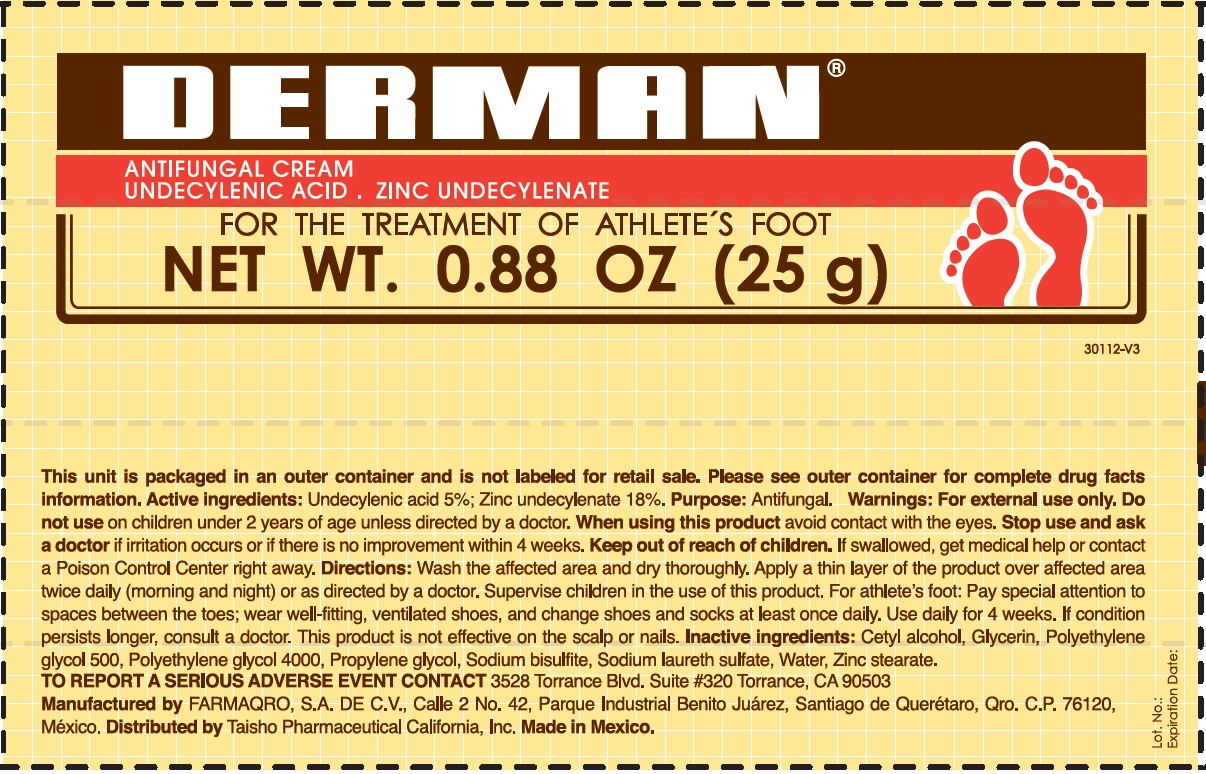
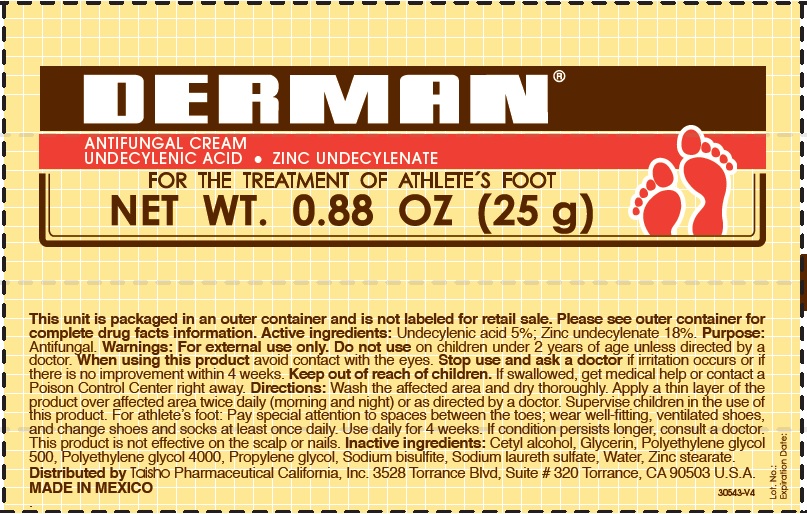
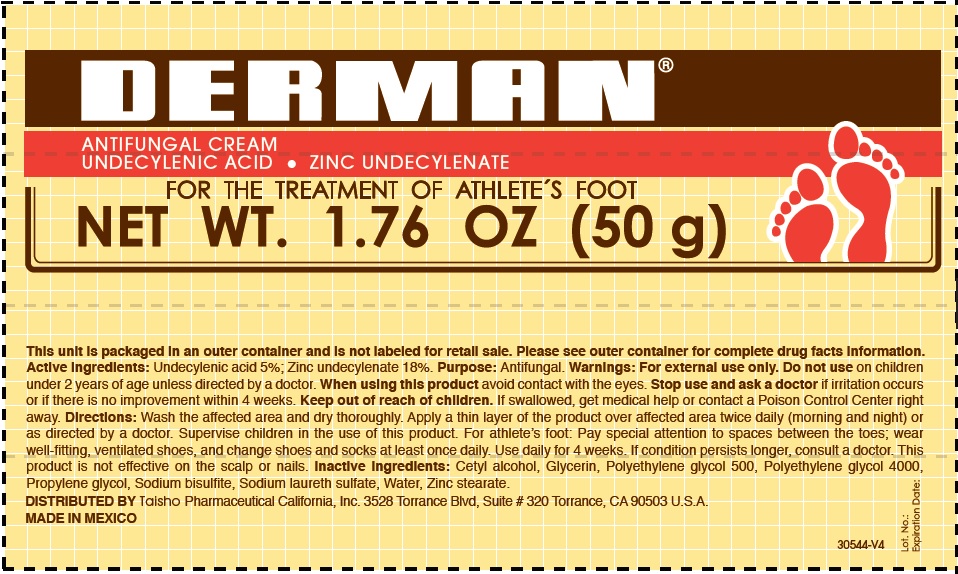
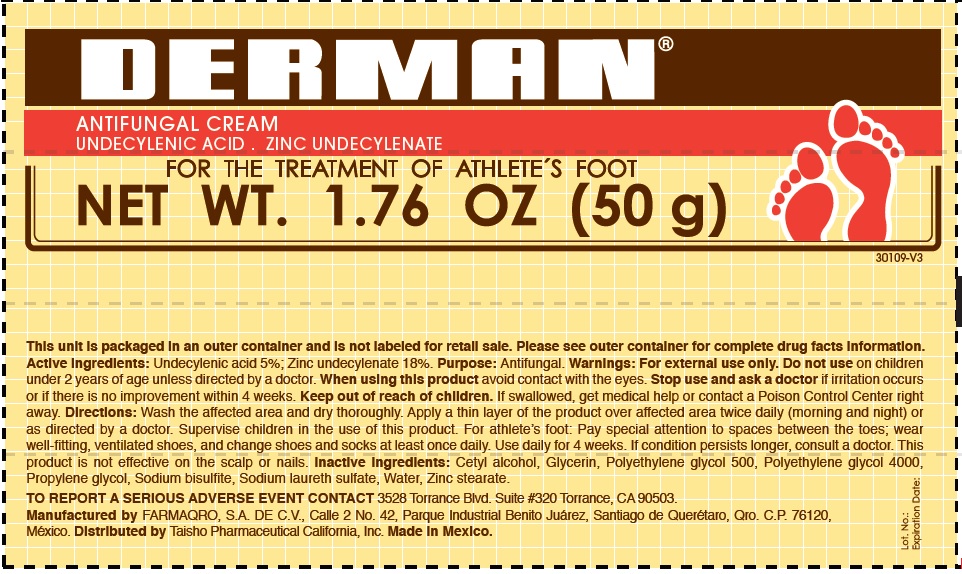
Label: DERMAN ANTIFUNGAL- zinc undecylenate cream
- NDC Code(s): 54312-125-01, 54312-125-02
- Packager: Compania Internacional de Comercio, S.A.P.I de C.V.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- Active Ingredients.
- Uses
- Warnings
-
Directions
- Wash the affected area and dry thoroughly.
- Apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor.
- Supervise children in the use of this product.
- For athlete's foot: Pay special attention to spaces between the toes; wear well fitting, ventilated shoes, and change shoes and socks at least once daily.
- Use daily for 4 weeks.
- If condition persists, consults a physician.
- This product is not effective on the scalp or nails.
- Inactive ingredients
- Derman Antifungal (54312-125-01)
- Derman Antifungal (54312-125-02)
-
INGREDIENTS AND APPEARANCE
DERMAN ANTIFUNGAL
zinc undecylenate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54312-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 5 g in 100 g ZINC UNDECYLENATE (UNII: 388VZ25DUR) (UNDECYLENIC ACID - UNII:K3D86KJ24N) ZINC UNDECYLENATE 18 g in 100 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 500 (UNII: 761NX2Q08Y) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BISULFITE (UNII: TZX5469Z6I) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) WATER (UNII: 059QF0KO0R) ZINC STEARATE (UNII: H92E6QA4FV) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54312-125-01 1 in 1 CARTON 08/12/2013 1 25 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:54312-125-02 1 in 1 CARTON 08/12/2013 2 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 08/12/2013 Labeler - Compania Internacional de Comercio, S.A.P.I de C.V. (822165213) Registrant - Compania Internacional de Comercio, S.A.P.I de C.V. (822165213) Establishment Name Address ID/FEI Business Operations Compania Internacional de Comercio, S.A.P.I de C.V. 822165213 manufacture(54312-125)