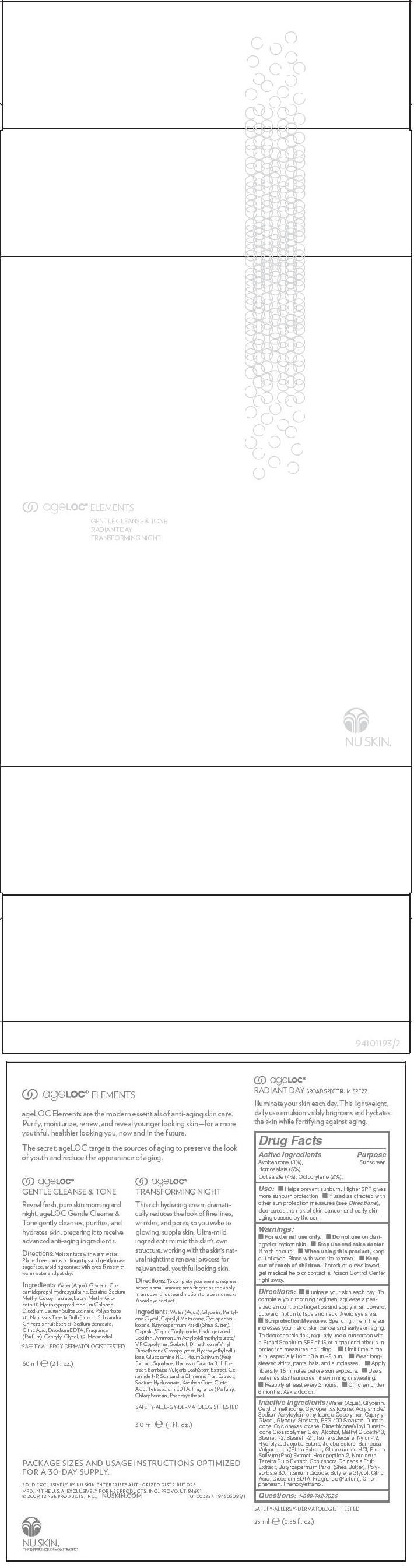
Label: AGELOC ELEMENTS- avobenzone, homosalate, octisalate, and octocrylene kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 62839-3887-1, 62839-3904-1 - Packager: NSE Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 20, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
-
Use
- Helps prevent sunburn. Higher SPF gives more sunburn protection
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Illuminate your skin each day. To complete your morning regimen, squeeze a pea-sized amount onto fingertips and apply in an upward, outward motion to face and neck. Avoid eye area.
-
Sunprotection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Water (Aqua), Glycerin, Cetyl Dimethicone, Cyclopentasiloxane, Acrylamide/Sodium Acryloyldimethyltaurate Copolymer, Caprylyl Glycol, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Cyclohexasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Cetyl Alcohol, Methyl Gluceth-10, Steareth-2, Steareth-21, Isohexadecane, Nylon-12, Hydrolyzed Jojoba Esters, Jojoba Esters, Bambusa Vulgaris Leaf/Stem Extract, Glucosamine HCl, Pisum Sativum (Pea) Extract, Hexapeptide-2, Narcissus Tazetta Bulb Extract, Schizandra Chinensis Fruit Extract, Butyrospermum Parkii (Shea Butter), Polysorbate 80, Titanium Dioxide, Butylene Glycol, Citric Acid, Disodium EDTA, Fragrance (Parfum), Chlorphenesin, Phenoxyethanol.
- Questions
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
AGELOC ELEMENTS
avobenzone, homosalate, octisalate, and octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62839-3887 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-3887-1 1 in 1 CARTON 05/01/2013 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE, WITH APPLICATOR 25 mL Part 2 1 BOTTLE, PUMP 60 mL Part 3 1 JAR 30 mL Part 1 of 3 NU SKIN AGELOC RADIANT DAY BROAD SPECTRUM SPF 22
avobenzone, homosalate, octisalate, and octocrylene lotionProduct Information Item Code (Source) NDC:62839-3904 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 mL Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 50 mg in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 40 mg in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Cyclomethicone 5 (UNII: 0THT5PCI0R) Caprylyl Glycol (UNII: 00YIU5438U) Glyceryl Monostearate (UNII: 230OU9XXE4) PEG-100 Stearate (UNII: YD01N1999R) Cetyl Alcohol (UNII: 936JST6JCN) Methyl Gluceth-10 (UNII: N0MWT4C7WH) Steareth-2 (UNII: V56DFE46J5) Steareth-21 (UNII: 53J3F32P58) Isohexadecane (UNII: 918X1OUF1E) Dimethicone (UNII: 92RU3N3Y1O) Nylon-12 (UNII: 446U8J075B) Phenoxyethanol (UNII: HIE492ZZ3T) Chlorphenesin (UNII: I670DAL4SZ) Cyclomethicone 6 (UNII: XHK3U310BA) Polysorbate 80 (UNII: 6OZP39ZG8H) Glucosamine Hydrochloride (UNII: 750W5330FY) Shea Butter (UNII: K49155WL9Y) Titanium Dioxide (UNII: 15FIX9V2JP) Pea (UNII: W4X7H8GYFM) Butylene Glycol (UNII: 3XUS85K0RA) Edetate Disodium (UNII: 7FLD91C86K) Bambusa Vulgaris Top (UNII: FIW80T6P6V) Narcissus Tazetta Bulb (UNII: K17762966S) Citric Acid Monohydrate (UNII: 2968PHW8QP) Schisandra Chinensis Fruit (UNII: ABS794681C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62839-3904-1 25 mL in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 05/01/2013 Part 2 of 3 AGELOC GENTLE CLEANSE AND TONE
cleansing (cold creams, cleansing lotions, liquids, and pads) liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR Glycerin (UNII: PDC6A3C0OX) INGR Cocamidopropyl Hydroxysultaine (UNII: 62V75NI93W) INGR Betaine (UNII: 3SCV180C9W) INGR Sodium Methyl Cocoyl Taurate (UNII: JVL98CG53G) INGR Disodium Laureth Sulfosuccinate (UNII: D6DH1DTN7E) INGR 1,2-Hexanediol (UNII: TR046Y3K1G) INGR Caprylyl Glycol (UNII: 00YIU5438U) INGR Polysorbate 20 (UNII: 7T1F30V5YH) INGR Sodium Benzoate (UNII: OJ245FE5EU) INGR Citric Acid Monohydrate (UNII: 2968PHW8QP) INGR Edetate Disodium (UNII: 7FLD91C86K) INGR Narcissus Tazetta Bulb (UNII: K17762966S) INGR Schisandra Chinensis Fruit (UNII: ABS794681C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 05/01/2013 Part 3 of 3 AGELOC TRANSFORMING NIGHT
night creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR Glycerin (UNII: PDC6A3C0OX) INGR Pentylene Glycol (UNII: 50C1307PZG) INGR Caprylyl Trisiloxane (UNII: Q95M2P1KJL) INGR Cyclomethicone 5 (UNII: 0THT5PCI0R) INGR Shea Butter (UNII: K49155WL9Y) INGR Medium-Chain Triglycerides (UNII: C9H2L21V7U) INGR Ammonium Acryloyldimethyltaurate/VP Copolymer (UNII: W59H9296ZG) INGR Sorbitol (UNII: 506T60A25R) INGR Chlorphenesin (UNII: I670DAL4SZ) INGR Hydroxymethyl Cellulose (UNII: 273FM27VK1) INGR Glucosamine Hydrochloride (UNII: 750W5330FY) INGR Squalane (UNII: GW89575KF9) INGR Xanthan Gum (UNII: TTV12P4NEE) INGR Pea (UNII: W4X7H8GYFM) INGR Edetate Sodium (UNII: MP1J8420LU) INGR Bambusa Vulgaris Top (UNII: FIW80T6P6V) INGR Narcissus Tazetta Bulb (UNII: K17762966S) INGR Citric Acid Monohydrate (UNII: 2968PHW8QP) INGR Schisandra Chinensis Fruit (UNII: ABS794681C) INGR Hyaluronate Sodium (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 05/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/01/2013 Labeler - NSE Products, Inc. (803486393)